Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation
Abstract
:1. Introduction
2. Results
2.1. Qualitative Nerve Analysis
2.2. Histomorphometric Nerve Analysis
2.3. Qualitative Muscle Analysis
2.4. Histomorphometric Muscle Analysis
2.5. Functional Analysis of Whiskers Movements
3. Discussion
4. Materials and Methods
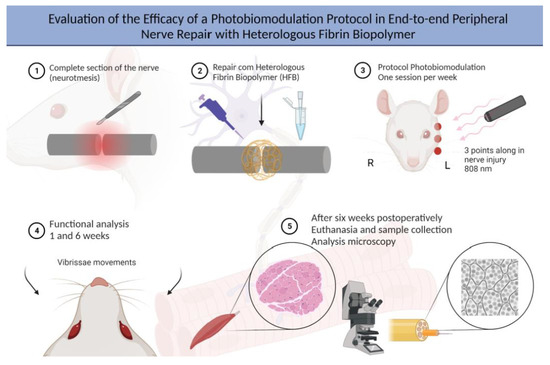
4.1. Experimental Design
4.2. Heterologous Fibrin Biopolymer (HFB)
4.3. Surgical Procedures
4.3.1. Denervation Surgery
4.3.2. Surgical Protocol for the Experimental Groups with Repair of the Buccal Branch of the Facial Nerve
4.3.3. Post-Surgical Care
4.4. Photobiomodulation Protocol (PBM)
4.5. Functional Analysis
4.6. Sample Collection and Euthanasia
4.7. Histological Processing of Nerve and Muscle
4.8. Histological Analysis of Nerve and Muscle
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, S.H.; Park, H.; Yoo, D.S.; Joo, W.; Rhoton, A. Microsurgical Anatomy of the Facial Nerve. Clin. Anat. 2021, 34, 90–102. [Google Scholar] [CrossRef]
- Stuzin, J.M.; Rohrich, R.J. Facial Nerve Danger Zones. Plast. Reconstr. Surg. 2020, 145, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.Q.; Tran Phan Chung, T.; Tran Viet, L.; Do Quang, H.; Tran Van, D.; Fox, A.J. The Anatomic Landmark Approach to Extratemporal Facial Nerve Repair in Facial Trauma. Cureus 2022, 14, e22787. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.K.; Lemoine, J.C.; Kahane, J.B.; Gary, C.C. Management of the Facial Nerve Following Temporal Bone Ballistic Injury. Laryngoscope Investig. Otolaryngol. 2022, 7, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.R.; Callahan, N.; Miloro, M. Management of Traumatic Trigeminal and Facial Nerve Injuries. Oral Maxillofac. Surg. Clin. North. Am. 2021, 33, 381–405. [Google Scholar] [CrossRef]
- Cho, Y.S.; Choi, J.E.; Lim, J.H.; Cho, Y.-S. Management of Facial Nerve Schwannoma: When Is the Timing for Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 1243–1249. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Silver, C.E.; Thielker, J.; Bernal-Sprekelsen, M.; Bradford, C.R.; de Bree, R.; Kowalski, L.P.; Olsen, K.D.; Quer, M.; Rinaldo, A.; et al. Management of the Facial Nerve in Parotid Cancer: Preservation or Resection and Reconstruction. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2615–2626. [Google Scholar] [CrossRef]
- Psillas, G.; Constantinidis, J. Facial Palsy Secondary to Cholesteatoma: A Case-Series of 14 Patients. Audiol. Res. 2023, 13, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zourntou, S.-E.; Makridis, K.G.; Tsougos, C.-I.; Skoulakis, C.; Vlychou, M.; Vassiou, A. Facial Nerve: A Review of the Anatomical, Surgical Landmarks and Its Iatrogenic Injuries. Injury 2021, 52, 2038–2048. [Google Scholar] [CrossRef]
- Green, J.D., Jr.; Shelton, C.; Brackmann, D.E. Iatrogenic facial nerve injury during otologic surgery. Laryngoscope 1994, 104, 922–926. [Google Scholar] [CrossRef]
- Spencer, C.R.; Irving, R.M. Causes and Management of Facial Nerve Palsy. Br. J. Hosp. Med. 2016, 77, 686–691. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, J.-E.; Yoon, B.-A.; Kim, J.-K.; Bae, J.-S. Bilateral Facial Weakness with Distal Paresthesia Following COVID-19 Vaccination: A Scoping Review for an Atypical Variant of Guillain–Barré Syndrome. Brain Sci. 2022, 12, 1046. [Google Scholar] [CrossRef]
- Jung, S.Y.; Jung, J.; Byun, J.Y.; Park, M.S.; Kim, S.H.; Yeo, S.G. The Effect of Metabolic Syndrome on Bell’s Palsy Recovery Rate. Acta Otolaryngol. 2018, 138, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Parsa, K.M.; Hancock, M.; Nguy, P.L.; Donalek, H.M.; Wang, H.; Barth, J.; Reilly, M.J. Association of Facial Paralysis with Perceptions of Personality and Physical Traits. JAMA Netw. Open 2020, 3, e205495. [Google Scholar] [CrossRef]
- Okuma, H.; Nagano, R.; Takagi, S. Hemiplegic Peripheral Neuropathy Accompanied with Multiple Cranial Nerve Palsy. Clin. Pract. 2012, 2, e40. [Google Scholar] [CrossRef] [Green Version]
- Li, M.K.K.; Niles, N.; Gore, S.; Ebrahimi, A.; McGuinness, J.; Clark, J.R. Social Perception of Morbidity in Facial Nerve Paralysis. Head Neck 2016, 38, 1158–1163. [Google Scholar] [CrossRef]
- Fliss, E.; Yanko, R.; Zaretski, A.; Tulchinsky, R.; Arad, E.; Kedar, D.J.; Fliss, D.M.; Gur, E. Facial Nerve Repair Following Acute Nerve Injury. Arch. Plast. Surg. 2022, 49, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Seddon, H.J. Three Types of Nerve Injury. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve Physiology. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, E.M.; Weber, R.V.; Mackinnon, S.E. Nerve Injury, Repair, and Reconstruction. In Plastic Surgery—Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2022; pp. 803–825. [Google Scholar]
- Riccio, M.; Marchesini, A.; Pugliese, P.; Francesco, F. Nerve Repair and Regeneration: Biological Tubulization Limits and Future Perspectives. J. Cell Physiol. 2019, 234, 3362–3375. [Google Scholar] [CrossRef]
- Grinsell, D.; Keating, C.P. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panagopoulos, G.N.; Megaloikonomos, P.D.; Mavrogenis, A.F. The Present and Future for Peripheral Nerve Regeneration. Orthopedics 2017, 40, e141–e156. [Google Scholar] [CrossRef]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef]
- Rönkkö, H.; Göransson, H.; Taskinen, H.-S.; Paavilainen, P.; Vahlberg, T.; Röyttä, M. Comparison of Peripheral Nerve Regeneration with Side-to-Side, End-to-Side, and End-to-End Repairs. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1179. [Google Scholar] [CrossRef]
- Battiston, B.; Artiaco, S.; Conforti, L.G.; Vasario, G.; Tos, P. End-to-Side Nerve Suture in Traumatic Injuries of Brachial Plexus: Review of the Literature and Personal Case Series. J. Hand Surg. Eur. Vol. 2009, 34, 656–659. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Khan, W. Recent Advances and Developments in Neural Repair and Regeneration for Hand Surgery. Open Orthop. J. 2012, 6, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Degrugillier, L.; Tremp, M.; Prautsch, K.; Sottaz, L.; Schaefer, D.J.; Madduri, S.; Kalbermatten, D. Nerve Repair With Fibrin Nerve Conduit and Modified Suture Placement. Anat. Rec. 2018, 301, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- Chow, N.; Miears, H.; Cox, C.; MacKay, B. Fibrin Glue and Its Alternatives in Peripheral Nerve Repair. Ann. Plast. Surg. 2021, 86, 103–108. [Google Scholar] [CrossRef]
- Bora, F.W.; Pleasure, D.E.; Didizian, N.A. A Study of Nerve Regeneration and Neuroma Formation after Nerve Suture by Various Techniques. J. Hand Surg. Am. 1976, 1, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.S.; Siqueira, M.G.; da Silva, C.F.; Plese, J.P.P. Overall Assessment of Regeneration in Peripheral Nerve Lesion Repair Using Fibrin Glue, Suture, or a Combination of the 2 Techniques in a Rat Model. Which Is the Ideal Choice? Surg. Neurol. 2005, 64, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Han, S.-G.; Kim, S.-H.; Zhu, S.-J.; Huh, J.-Y.; Jung, J.-H.; Lee, S.-H.; Kim, B.-Y. Autologous Fibrin Glue in Peripheral Nerve Regeneration in Vivo. Microsurgery 2005, 25, 495–499. [Google Scholar] [CrossRef]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; dos Santos, L.D.; Barraviera, B. Heterologous Fibrin Sealant Derived from Snake Venom: From Bench to Bedside—An Overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.C.; Ferreira, R.S.; Barraviera, S.R.C.S.; Stolf, H.O.; Thomazini-Santos, I.A.; Mendes-Giannini, M.J.S.; Toscano, E.; Barraviera, B. A New Fibrin Sealant From Crotalus Durissus Terrificus Venom: Applications in Medicine. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Vieira, L.; Barraviera, B.; Barraviera, S. Treatment of Venous Ulcers with Fibrin Sealant Derived from Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 226–229. [Google Scholar] [CrossRef]
- Leite, A.P.S.; Pinto, C.G.; Tibúrcio, F.C.; Sartori, A.A.; de Castro Rodrigues, A.; Barraviera, B.; Ferreira, R.S.; Filadelpho, A.L.; Matheus, S.M.M. Heterologous Fibrin Sealant Potentiates Axonal Regeneration after Peripheral Nerve Injury with Reduction in the Number of Suture Points. Injury 2019, 50, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; de Rodrigues, A.C.; de Macedo, M.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- De Rosso, M.P.O.; Rosa Júnior, G.M.; Buchaim, D.V.; German, I.J.S.; Pomini, K.T.; de Souza, R.G.; Pereira, M.; Favaretto Júnior, I.A.; de Souza Bueno, C.R.; de Oliveira Gonçalves, J.B.; et al. Stimulation of Morphofunctional Repair of the Facial Nerve with Photobiomodulation, Using the End-to-Side Technique or a New Heterologous Fibrin Sealant. J. Photochem. Photobiol. B 2017, 175, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.I.; Gurjar, A.A.; Talukder, M.A.H.; Rodenhouse, A.; Manto, K.; O’Brien, M.; Govindappa, P.K.; Elfar, J.C. A Novel Nerve Transection and Repair Method in Mice: Histomorphometric Analysis of Nerves, Blood Vessels, and Muscles with Functional Recovery. Sci. Rep. 2020, 10, 21637. [Google Scholar] [CrossRef] [PubMed]
- de Bueno, C.R.S.; Pereira, M.; Favaretto Junior, I.A.; Bortoluci, C.H.F.; dos Santos, T.C.P.; Dias, D.V.; Daré, L.R.; Rosa Junior, G.M. Electrical Stimulation Attenuates Morphological Alterations and Prevents Atrophy of the Denervated Cranial Tibial Muscle. Einstein 2017, 15, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, J.S.F.; Marques, M.J.; Macedo, A.B.; de Carvalho, S.C.; Neto, H.S. Effect of Photobiomodulation on Denervation-Induced Skeletal Muscle Atrophy and Autophagy: A Study in Mice. J. Manip. Physiol. Ther. 2022, 45, 97–103. [Google Scholar] [CrossRef]
- Pasquale, C.; Utyuzh, A.; Mikhailova, M.V.; Colombo, E.; Amaroli, A. Recovery from Idiopathic Facial Paralysis (Bell’s Palsy) Using Photobiomodulation in Patients Non-Responsive to Standard Treatment: A Case Series Study. Photonics 2021, 8, 341. [Google Scholar] [CrossRef]
- Hakimiha, N.; Rokn, A.R.; Younespour, S.; Moslemi, N. Photobiomodulation Therapy for the Management of Patients With Inferior Alveolar Neurosensory Disturbance Associated With Oral Surgical Procedures: An Interventional Case Series Study. J. Lasers Med. Sci. 2020, 11, S113–S118. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.J.; Fazan, V.P.S.; Cury, D.P.; de Almeida, S.R.Y.; Borie, E.; Fuentes, R.; Coutinho-Netto, J.; Watanabe, I. Growth Factors Expression and Ultrastructural Morphology after Application of Low-Level Laser and Natural Latex Protein on a Sciatic Nerve Crush-Type Injury. PLoS ONE 2019, 14, e0210211. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.; Buchaim, D.; Kawano, N.; Furlanette, G.; Pomini, K.; Buchaim, R. Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review. Bioengineering 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigo-Benato, D.; Russo, T.L.; Tanaka, E.H.; Assis, L.; Salvini, T.F.; Parizotto, N.A. Effects of 660 and 780 Nm Low-Level Laser Therapy on Neuromuscular Recovery after Crush Injury in Rat Sciatic Nerve. Lasers Surg. Med. 2010, 42, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Andraus, R.A.C.; Maia, L.P.; de Souza Lino, A.D.; Fernandes, K.B.P.; de Matos Gomes, M.V.; de Jesus Guirro, R.R.; Barbieri, C.H. LLLT Actives MMP-2 and Increases Muscle Mechanical Resistance after Nerve Sciatic Rat Regeneration. Lasers Med. Sci. 2017, 32, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum-Livnat, M.M.; Almog, M.; Nissan, M.; Loeb, E.; Shapira, Y.; Rochkind, S. Photobiomodulation Triple Treatment in Peripheral Nerve Injury: Nerve and Muscle Response. Photomed. Laser Surg. 2016, 34, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Geuna, S.; Shainberg, A. Phototherapy and nerve injury: Focus on muscle response. Int. Rev. Neurobiol. 2013, 109, 99–109. [Google Scholar] [CrossRef]
- Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; de Oliveira, A.L.R.; de Castro Rodrigues, A. Effect of Low-Level Laser Therapy (LLLT) on Peripheral Nerve Regeneration Using Fibrin Glue Derived from Snake Venom. Injury 2015, 46, 655–660. [Google Scholar] [CrossRef]
- de Faria, S.D.; Testa, J.R.G.; Borin, A.; Toledo, R.N. Standardization of Techniques Used in Facial Nerve Section and Facial Movement Evaluation in Rats. Braz. J. Otorhinolaryngol. 2006, 72, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Koopman, J.E.; Duraku, L.S.; de Jong, T.; de Vries, R.B.M.; Michiel Zuidam, J.; Hundepool, C.A. A Systematic Review and Meta-Analysis on the Use of Fibrin Glue in Peripheral Nerve Repair: Can We Just Glue It? J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1018–1033. [Google Scholar] [CrossRef] [PubMed]
- Sameem, M.; Wood, T.J.; Bain, J.R. A Systematic Review on the Use of Fibrin Glue for Peripheral Nerve Repair. Plast. Reconstr. Surg. 2011, 127, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, S.; Vila-Torres, J.; Lorenzo, J. Fibrin Glue Is A Sealant and Not a Nerve Barrier. J. Reconstr. Microsurg. 1995, 11, 135–139. [Google Scholar] [CrossRef]
- Rafijah, G.; Bowen, A.J.; Dolores, C.; Vitali, R.; Mozaffar, T.; Gupta, R. The Effects of Adjuvant Fibrin Sealant on the Surgical Repair of Segmental Nerve Defects in an Animal Model. J. Hand Surg. Am. 2013, 38, 847–855. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral Nerve Injury and Myelination: Potential Therapeutic Strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- della Santa, G.M.L.; Ferreira, M.C.; Machado, T.P.G.; Oliveira, M.X.; Santos, A.P. Effects of Photobiomodulation Therapy (LED 630 Nm) on Muscle and Nerve Histomorphometry after Axonotmesis. Photochem. Photobiol. 2021, 97, 1116–1122. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral Nerve Regeneration: Experimental Strategies and Future Perspectives. Adv. Drug. Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Andreo, L.; Soldera, C.B.; Ribeiro, B.G.; de Matos, P.R.V.; Bussadori, S.K.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A. Effects of Photobiomodulation on Experimental Models of Peripheral Nerve Injury. Lasers Med. Sci. 2017, 32, 2155–2165. [Google Scholar] [CrossRef]
- Lee, J.; Carpena, N.T.; Kim, S.; Lee, M.Y.; Jung, J.Y.; Choi, J.E. Photobiomodulation at a Wavelength of 633 Nm Leads to Faster Functional Recovery than 804 Nm after Facial Nerve Injury. J. Biophotonics 2021, 14, e202100159. [Google Scholar] [CrossRef]
- Li, B.; Wang, X. Photobiomodulation Enhances Facial Nerve Regeneration via Activation of PI3K/Akt Signaling Pathway–Mediated Antioxidant Response. Lasers Med. Sci. 2022, 37, 993–1006. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Geuna, S.; Raimondo, S.; Ronchi, G.; di Scipio, F.; Tos, P.; Czaja, K.; Fornaro, M. Chapter 3 Histology of the Peripheral Nerve and Changes Occurring During Nerve Regeneration. Int. Rev. Neurobiol. 2009, 87, 27–46. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, S.E.; Dellon, A.L.; O’Brien, J.P. Changes in Nerve Fiber Numbers Distal to a Nerve Repair in the Rat Sciatic Nerve Model. Muscle Nerve 1991, 14, 1116–1222. [Google Scholar] [CrossRef]
- Wang, M.L.; Rivlin, M.; Graham, J.G.; Beredjiklian, P.K. Peripheral Nerve Injury, Scarring, and Recovery. Connect. Tissue. Res. 2019, 60, 3–9. [Google Scholar] [CrossRef]
- Fu, T.; Jiang, L.; Peng, Y.; Li, Z.; Liu, S.; Lu, J.; Zhang, F.; Zhang, J. Electrical Muscle Stimulation Accelerates Functional Recovery after Nerve Injury. Neuroscience 2020, 426, 179–188. [Google Scholar] [CrossRef]
- Chu, X.-L.; Song, X.-Z.; Li, Q.; Li, Y.-R.; He, F.; Gu, X.-S.; Ming, D. Basic Mechanisms of Peripheral Nerve Injury and Treatment via Electrical Stimulation. Neural Regen. Res. 2022, 17, 2185–2193. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.; Shao, C.; Liu, J.; Ding, F.; Gu, X. Expression Pattern of Myostatin in Gastrocnemius Muscle of Rats after Sciatic Nerve Crush Injury. Muscle Nerve 2007, 35, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Angelov, D.N.; Ceynowa, M.; Guntinas-Lichius, O.; Streppel, M.; Grosheva, M.; Kiryakova, S.I.; Skouras, E.; Maegele, M.; Irintchev, A.; Neiss, W.F.; et al. Mechanical Stimulation of Paralyzed Vibrissal Muscles Following Facial Nerve Injury in Adult Rat Promotes Full Recovery of Whisking. Neurobiol. Dis. 2007, 26, 229–242. [Google Scholar] [CrossRef]
- Pinto, M.M.R.; dos Santos, D.R.; de Barros Bentes, L.G.; Lemos, R.S.; de Almeida, N.R.C.; Fernandes, M.R.N.; Braga, J.P.; Somensi, D.N.; Barros, R.S.M. Anatomical Description of the Extratemporal Facial Nerve under High-Definition System: A Microsurgical Study in Rats. Acta Cir. Bras. 2022, 37, e370803. [Google Scholar] [CrossRef]
- DeLeonibus, A.; Rezaei, M.; Fahradyan, V.; Silver, J.; Rampazzo, A.; Bassiri Gharb, B. A meta-analysis of Functional Outcomes in Rat Sciatic Nerve Injury Models. Microsurgery 2021, 41, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.; Hazel, A.; Palispis, W.; Suryadevara, S.; Gupta, R. Functional Assessment after Sciatic Nerve Injury in a Rat Model. Microsurgery 2009, 29, 644–649. [Google Scholar] [CrossRef]
- Yian, C.H.; Paniello, R.C.; Gershon Spector, J. Inhibition of Motor Nerve Regeneration in a Rabbit Facial Nerve Model. Laryngoscope 2001, 111, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, H.; Bi, W.; Tan, X.; Li, R.; Wen, W.; Song, W.; Zhang, Y.; Zhang, F.; Hu, M. Effect of Chitosan Combined with Hyaluronate on Promoting the Recovery of Postoperative Facial Nerve Regeneration and Function in Rabbits. Exp. Ther. Med. 2018, 16, 739–745. [Google Scholar] [CrossRef]
- de Bueno, C.R.S.; Pereira, M.; Favaretto-Júnior, I.A.; Buchaim, R.L.; Andreo, J.C.; Rodrigues, A.d.C.; Rosa-Júnior, G.M. Comparative Study between Standard and Inside-out Vein Graft Techniques on Sciatic Nerve Repair of Rats. Muscular and Functional Analysis. Acta Cir. Bras. 2017, 32, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthou, M.E.; Gencheva, D.; Sinis, N.; Rink, S.; Papamitsou, T.; Abdulla, D.; Bendella, H.; Sarikcioglu, L.; Angelov, D.N. Facial Nerve Repair by Muscle-Vein Conduit in Rats: Functional Recovery and Muscle Reinnervation. Tissue Eng. Part A 2021, 27, 351–361. [Google Scholar] [CrossRef]
- Viterbo, F.; Brock, R.S.; Maciel, F.; Ayestaray, B.; Garbino, J.A.; Rodrigues, C.P. End-to-Side versus End-to-End Neurorrhaphy at the Peroneal Nerve in Rats. Acta Cir. Bras. 2017, 32, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, O.A.R.; Gordon, T. A Rat Study of the Use of End-to-Side Peripheral Nerve Repair as a “Babysitting” Technique to Reduce the Deleterious Effect of Chronic Denervation. J. Neurosurg. 2019, 131, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Kouyoumdjian, J.; Graç, C.; Ferreira, V.M. Peripheral Nerve Injuries: A Retrospective Survey of 1124 Cases. Neurol. India 2017, 65, 551–555. [Google Scholar] [CrossRef]
- Kouyoumdjian, J.A. Peripheral Nerve Injuries: A Retrospective Survey of 456 Cases. Muscle Nerve 2006, 34, 785–788. [Google Scholar] [CrossRef]
- Ronchi, G.; Cillino, M.; Gambarotta, G.; Fornasari, B.E.; Raimondo, S.; Pugliese, P.; Tos, P.; Cordova, A.; Moschella, F.; Geuna, S. Irreversible Changes Occurring in Long-Term Denervated Schwann Cells Affect Delayed Nerve Repair. J. Neurosurg. 2017, 127, 843–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Buchaim, D.V.; de Rodrigues, A.C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.F.; Junior, G.M.R.; de Souza Bueno, C.R.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The New Heterologous Fibrin Sealant in Combination with Low-Level Laser Therapy (LLLT) in the Repair of the Buccal Branch of the Facial Nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibúrcio, F.C.; Muller, K.S.; Leite, A.P.S.; de Oliveira, I.R.A.; Barraviera, B.; Ferreira, R.S., Jr.; Padovani, C.R.; Pinto, C.G.; Matheus, S.M.M. Neuroregeneration and immune response after neurorrhaphy are improved with the use of heterologous fibrin biopolymer in addition to suture repair alone. Muscle Nerve 2023. ahead of print. [Google Scholar] [CrossRef]
- Pinto, C.G.; Leite, A.P.S.; Sartori, A.A.; Tibúrcio, F.C.; Barraviera, B.; Junior, R.S.F.; Filadelpho, A.L.; de Carvalho, S.C.; Matheus, S.M.M. Heterologous fibrin biopolymer associated to a single suture stitch enables the return of neuromuscular junction to its mature pattern after peripheral nerve injury. Injury 2021, 52, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Lima, A.B.B.C.O.; Haddad, G.R.; Gatti, M.A.N.; Medolago, N.B.; Rigotto Carneiro, M.T.; dos Santos, L.D.; Ferreira, R.S.; et al. Treatment of Chronic Venous Ulcers With Heterologous Fibrin Sealant: A Phase I/II Clinical Trial. Front. Immunol. 2021, 12, 627541. [Google Scholar] [CrossRef]
- Daré, L.R.; Dias, D.V.; Rosa Junior, G.M.; Bueno, C.R.S.; Buchaim, R.L.; Rodrigues, A.d.C.; Andreo, J.C. Effect of β-Hydroxy-β-Methylbutyrate in Masticatory Muscles of Rats. J. Anat. 2015, 226, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Groups | Fiber Area (µm2) | Axon Area (µm2) | Fiber Diameter (µm) | Axon Diameter (µm) | Myelin Sheath Area (µm2) | Myelin Sheath Thickness (µm) |
|---|---|---|---|---|---|---|
| CGn | 56.11 ± 1.93 | 17.27 ± 1.07 | 8.53 ± 0.28 | 4.36 ± 0,10 | 39.65 ± 0,70 | 4.16 ± 0.24 |
| CGl | 58.37 ± 3.66 | 18.17 ± 0.87 | 9.21 ± 0.43 | 4.55 ± 0.33 | 40,74 ± 2.81 | 4.49 ± 0.42 |
| ERGn | 38.77 ± 2.96 | 14.28 ± 0.77 | 7.10 ± 0.25 | 3.31 ± 0.19 | 24.57 ± 0.57 | 3.73 ± 0.08 |
| ERGl | 41.77 ± 1.50 | 14.97 ± 0.28 | 8.00 ± 0.36 | 4.07 ± 0.27 | 27.13 ± 1.46 | 3.95 ± 0.09 |
| Groups | Fiber Muscle Area (µm2) |
|---|---|
| CGn | 1126.00 ± 250.50 |
| CGl | 1208.00 ± 249.60 |
| DGn | 603.60 ± 85.54 |
| DGl | 583.00 ± 44.36 |
| ERGn | 837.30 ± 113.80 |
| ERGl | 926.90 ± 183.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, C.R.d.S.; Tonin, M.C.C.; Buchaim, D.V.; Barraviera, B.; Ferreira Junior, R.S.; Santos, P.S.d.S.; Reis, C.H.B.; Pastori, C.M.; Pereira, E.d.S.B.M.; Nogueira, D.M.B.; et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals 2023, 16, 653. https://doi.org/10.3390/ph16050653
Bueno CRdS, Tonin MCC, Buchaim DV, Barraviera B, Ferreira Junior RS, Santos PSdS, Reis CHB, Pastori CM, Pereira EdSBM, Nogueira DMB, et al. Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation. Pharmaceuticals. 2023; 16(5):653. https://doi.org/10.3390/ph16050653
Chicago/Turabian StyleBueno, Cleuber Rodrigo de Souza, Maria Clara Cassola Tonin, Daniela Vieira Buchaim, Benedito Barraviera, Rui Seabra Ferreira Junior, Paulo Sérgio da Silva Santos, Carlos Henrique Bertoni Reis, Cláudio Maldonado Pastori, Eliana de Souza Bastos Mazuqueli Pereira, Dayane Maria Braz Nogueira, and et al. 2023. "Morphofunctional Improvement of the Facial Nerve and Muscles with Repair Using Heterologous Fibrin Biopolymer and Photobiomodulation" Pharmaceuticals 16, no. 5: 653. https://doi.org/10.3390/ph16050653