Controlled Drug Delivery Device for Cornea Treatment and Novel Method for Its Testing
Abstract
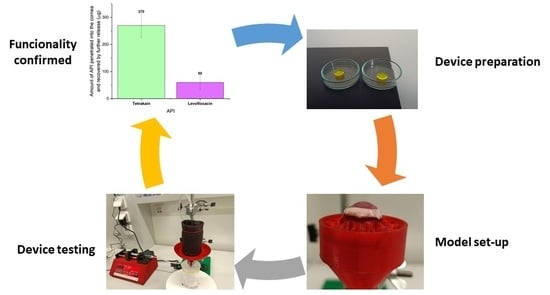
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Drug Carrier
2.2. Drug Release from the Collagen Carrier
2.3. Verification of Riboflavin Removal from the Crosslinked Barrier
2.4. Chemical Stability of API
3. Materials and Methods
3.1. Materials
3.2. UV-Vis Analysis
3.3. Drug Carrier Preparation
3.4. Model Device and Drug Release Experiment
3.5. HPLC Analysis of API
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Wong, Y.L.; Walkden, A. Current Perspectives on Corneal Transplantation. Clin. Ophthalmol. 2022, 16, 631–646. [Google Scholar] [CrossRef]
- Nonpassopon, M.; Niparugs, M.; Cortina, M.S. Boston Type 1 Keratoprosthesis: Updated Perspectives. Clin. Ophthalmol. 2020, 14, 1189–1200. [Google Scholar] [CrossRef]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 2145. [Google Scholar] [CrossRef]
- McKay, T.B.; Seyed-Razavi, Y.; Ghezzi, C.E.; Dieckmann, G.; Nieland, T.J.F.; Cairns, D.M.; Pollard, R.E.; Hamrah, P.; Kaplan, D.L. Corneal pain and experimental model development. Prog. Retin. Eye Res. 2019, 71, 88–113. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2014, 23, 1075–1091. [Google Scholar] [CrossRef]
- Sripetch, S.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: Thermodynamic considerations. Int. J. Pharm. 2021, 597, 120332. [Google Scholar] [CrossRef]
- Bennett, N.H.; Chinnery, H.R.; Downie, L.E.; Hill, L.J.; Grover, L.M. Material, Immunological, and Practical Perspectives on Eye Drop Formulation. Adv. Funct. Mater. 2020, 30, 1908476. [Google Scholar] [CrossRef]
- Colomé-Campos, J.; Martínez-Salcedo, I.; Martorell-Hallado, M.C.; Romero-Aroca, P. Objective evaluation of applying eye drops by elderly patients. Arch. Soc. Española Oftalmol. 2014, 89, 177–181. [Google Scholar] [CrossRef]
- Luo, C.; Wang, H.; Chen, X.; Xu, J.; Yin, H.; Yao, K. Recent Advances of Intraocular Lens Materials and Surface Modification in Cataract Surgery. Front. Bioeng. Biotechnol. 2022, 10, 996. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Singhania, S.S.; Desai, A.R.; Shukla, M.R.; Tannk, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Contact lenses with dual drug delivery for the treatment of bacterial conjunctivitis. Int. J. Pharm. 2018, 548, 139–150. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Fonseca, B.; Carracedo, G.; Martin-Gil, A.; Martinez-Aguila, A.; Pintor, J. Dry Eye Treatment Based on Contact Lens Drug Delivery: A Review. Eye Contact Lens 2016, 42, 280–288. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-loaded silicone hydrogels as contact lenses to address diabetic-eye complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef]
- Brooks, C.C.; Jabbehdari, S.; Gupta, P.K. Dexamethasone 0.4mg Sustained-Release Intracanalicular Insert in the Management of Ocular Inflammation and Pain Following Ophthalmic Surgery: Design, Development and Place in Therapy. Clin. Ophthalmol. 2020, 14, 89–94. [Google Scholar] [CrossRef]
- Tyson, S.L.; Bafna, S.; Gira, J.P.; Goldberg, D.F.; Jones, J.J.; Jones, M.P.; Kim, J.K.; Martel, J.M.; Nordlund, M.L.; Piovanetti-Perez, I.K.; et al. Multicenter randomized phase 3 study of a sustained-release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. J. Cataract Refract. Surg. 2019, 45, 204–212. [Google Scholar] [CrossRef]
- McCabe, C.; Desai, P.; Nijm, L.; Osher, R.; Weinstock, R. Real-World Experience with Intracapsular Administration of Dexamethasone Intraocular Suspension 9% for Control of Postoperative Inflammation. Clin. Ophthalmol. 2022, 16, 1985–1992. [Google Scholar] [CrossRef]
- Baig, M.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box–Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: Optimization, in vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef]
- Baran-Rachwalska, P.; Torabi-Pour, N.; Sutera, F.M.; Ahmed, M.; Thomas, K.; Nesbit, M.A.; Welsh, M.; Moore, C.B.T.; Saffie-Siebert, S.R. Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles. J. Control. Release 2020, 326, 192–202. [Google Scholar] [CrossRef]
- Wu, T.; Hou, X.; Li, J.; Ruan, H.; Pei, L.; Guo, T.; Wang, Z.; Ci, T.; Ruan, S.; He, Y.; et al. Microneedle-Mediated Biomimetic Cyclodextrin Metal Organic Frameworks for Active Targeting and Treatment of Hypertrophic Scars. ACS Nano 2021, 15, 20087–20104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, K.; Li, Z.; Guo, T.; Wu, T.; Hou, X.; Feng, N. Co-hybridized composite nanovesicles for enhanced transdermal eugenol and cinnamaldehyde delivery and their potential efficacy in ulcerative colitis. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102212. [Google Scholar] [CrossRef]
- Shi, Y.; Bikkuzin, T.; Song, Z.; Jin, X.; Jin, H.; Li, X.; Zhang, H. Comprehensive evaluation of decellularized porcine corneal after clinical transplantation. Xenotransplantation 2017, 24, e12338. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y.; Polarek, J.W.; Söderqvist, M.; Griffith, M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-Month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2010, 2, 46ra61. [Google Scholar] [CrossRef]
- Ong, J.A.; Auvinet, E.; Forget, K.J.; Lagali, N.; Fagerholm, P.; Griffith, M.; Meunier, J.; Brunette, I. 3D Corneal Shape After Implantation of a Biosynthetic Corneal Stromal Substitute. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2355–2365. [Google Scholar] [CrossRef]
- Lagali, N.; Fagerholm, P.; Griffith, M. Biosynthetic corneas: Prospects for supplementing the human donor cornea supply. Expert Rev. Med. Devices 2011, 8, 127–130. [Google Scholar] [CrossRef]
- El-Massry, A.; Ibrahim, O.; Abdalla, M.; Osman, I.; Mahmoud, S. Safety and Indicative Effectiveness of Porcine Corneal Lenticular Implants in Patients with Advanced Keratoconus and Post Lasik Ectasia: A Retrospective Clinical Study. Clin. Ophthalmol. 2021, 15, 3165–3171. [Google Scholar] [CrossRef]
- Khodaparast, M.; Shahraki, K.; Jabbarvand, M.; Shahraki, K.; Rafat, M.; Moravvej, Z. Sutureless Femtosecond Laser-Assisted Anterior Lamellar Keratoplasty Using a Bioengineered Cornea as a Viable Alternative to Human Donor Transplantation for Superficial Corneal Opacities. Cornea 2020, 39, 1184–1189. [Google Scholar] [CrossRef]
- Lombardo, M.; Micali, N.; Villari, V.; Serrao, S.; Pucci, G.; Barberi, R.; Lombardo, G. Ultraviolet A: Visible spectral absorbance of the human cornea after transepithelial soaking with dextran-enriched and dextran-free riboflavin 0.1% ophthalmic solutions. J. Cataract Refract. Surg. 2015, 41, 2283–2290. [Google Scholar] [CrossRef]
- Raiskup, F.; Spoerl, E. Corneal Crosslinking with Riboflavin and Ultraviolet A. I. Principles. Ocul. Surf. 2013, 11, 65–74. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of Collagen Crosslinking in Diabetes and Keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef]
- Scott McCall, A.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.C.; Clement, E.M.; Conrad, G.W. Mechanisms of Corneal Tissue Cross-linking in Response to Treatment with Topical Riboflavin and Long-Wavelength Ultraviolet Radiation (UVA). Investig. Ophthalmol. Vis. Sci. 2010, 51, 129–138. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Wollensak, G.; Spoerl, E.; Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J. Cataract Refract. Surg. 2003, 29, 1780–1785. [Google Scholar] [CrossRef]
- Wilson, A.; Jones, J.; Marshall, J. Biomechanical Evaluation of Decellularized and Crosslinked Corneal Implants Manufactured From Porcine Corneas as a Treatment Option for Advanced Keratoconus. Front. Bioeng. Biotechnol. 2022, 10, 491. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Islam, M.M.; Abusamra, D.B.; Chivu, A.; Argüeso, P.; Dohlman, C.H.; Patra, H.K.; Chodosh, J.; González-Andrades, M. Optimization of Collagen Chemical Crosslinking to Restore Biocompatibility of Tissue-Engineered Scaffolds. Pharmaceutics 2021, 13, 832. [Google Scholar] [CrossRef]
- Koh, L.B.; Islam, M.M.; Mitra, D.; Noel, C.W.; Merrett, K.; Odorcic, S.; Fagerholm, P.; Jackson, W.B.; Liedberg, B.; Phopase, J.; et al. Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. J. Funct. Biomater. 2013, 4, 162–177. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Aung, T.T.; Murugan, E.; Chen, L.; Lwin, N.C.; Zhou, L.; Beuerman, R.W.; Liedberg, B.; Venkatraman, S.S.; et al. Collagen-Based Artificial Corneal Scaffold with Anti-Infective Capability for Prevention of Perioperative Bacterial Infections. ACS Biomater. Sci. Eng. 2015, 1, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Chivu, A.; AbuSamra, D.B.; Saha, A.; Chowdhuri, S.; Pramanik, B.; Dohlman, C.H.; Das, D.; Argüeso, P.; Rajaiya, J.; et al. Crosslinker-free collagen gelation for corneal regeneration. Sci. Rep. 2022, 12, 9108. [Google Scholar] [CrossRef]
- Regal, S.; O’Connor, D.; Brige, P.; Delattre, R.; Djenizian, T.; Ramuz, M. Determination of optical parameters of the porcine eye and development of a simulated model. J. Biophotonics 2019, 12, e201800398. [Google Scholar] [CrossRef]
- Netto, A.R.T.; Hurst, J.; Bartz-Schmidt, K.-U.; Schnichels, S.; Rocha, A.; Netto, T.; Hurst, J.; Bartz-Schmidt, K.-U.; Schnichels, S. Porcine Corneas Incubated at Low Humidity Present Characteristic Features Found in Dry Eye Disease. Int. J. Mol. Sci. 2022, 23, 4567. [Google Scholar] [CrossRef] [PubMed]
- Danielewska, M.E.; Kostyszak, M.A.; Sareło, P.; Gąsior-Głogowska, M.; Niemczyk, M.; Prządka, P.; Antończyk, A.; Kiełbowicz, Z.; Iskander, D.R. Indirectly assessing changes in corneal properties with OCT speckle after crosslinking in porcine eyes. Exp. Eye Res. 2022, 219, 109051. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular delivery of cyclosporine A using dissolvable microneedle contact lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Choy, E.P.Y.; Cho, P.; Benzie, I.F.F.; Choy, C.K.M.; To, T.S.S. A novel porcine dry eye model system (pDEM) with simulated lacrimation/blinking system: Preliminary findings on system variability and effect of corneal drying. Curr. Eye Res. 2004, 28, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Cho, P.; Boost, M. Corneal epithelial cell viability of an ex vivo porcine eye model. Clin. Exp. Optom. 2014, 97, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, E.; Mrochen, M.; Sliney, D.; Trokel, S.; Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007, 26, 385–389. [Google Scholar] [CrossRef]
- Wu, D.; Lim, D.K.A.; Lim, B.X.H.; Wong, N.; Hafezi, F.; Manotosh, R.; Lim, C.H.L. Corneal Cross-Linking: The Evolution of Treatment for Corneal Diseases. Front. Pharmacol. 2021, 12, 686630. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Miura, J.; Ishimoto, T.; Yagi, K.; Matsuda, Y.; Shimizu, M.; Nakano, T.; Hayashi, M. UVA-activated riboflavin promotes collagen crosslinking to prevent root caries. Sci. Rep. 2019, 9, 1252. [Google Scholar] [CrossRef]
- Ahmadi, S.S.; Besharat, M.A.; Azizi, K.; Larijani, R. The Relationship Between Dimensions of Anger and Aggression in Contact and Noncontact Sports. Procedia Soc. Behav. Sci. 2011, 30, 247–251. [Google Scholar] [CrossRef]
- Kokaislová, A.; Kalhousová, M.; Gráfová, M.; Matějka, P. Chemometric evaluation of temperature-dependent surface-enhanced Raman spectra of riboflavin: What is the best multivariate approach to describe the effect of temperature? J. Mol. Struct. 2014, 1075, 609–619. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wagner, M.; Horn, H.; Niessner, R.; Haisch, C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal. Bioanal. Chem. 2009, 393, 197–206. [Google Scholar] [CrossRef]
- Suzuki, S.; Gerner, P.; Lirk, P. Local Anesthetics, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323481106. [Google Scholar]
- Haas, D.A.; Quinn, C.L. Local Anesthetics, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323393072. [Google Scholar]
- Mu, S.; Wang, C.; Liu, H.; Han, G.; Wu, L.; Li, J. Development and evaluation of a novelty single-step cleanup followed by HPLC-QTRAP-MS/MS for rapid analysis of tricaine, tetracaine, and bupivacaine in fish samples. Biomed. Chromatogr. 2021, 35, e5176. [Google Scholar] [CrossRef]
- Szerkus, O.; Jacyna, J.; Gibas, A.; Sieczkowski, M.; Siluk, D.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Robust HPLC–MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue. J. Pharm. Biomed. Anal. 2017, 132, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbánek, P.; Šuly, P.; Ševčík, J.; Hanulíková, B.; Kuřitka, I.; Šopík, T.; Stodůlka, P. Controlled Drug Delivery Device for Cornea Treatment and Novel Method for Its Testing. Pharmaceuticals 2023, 16, 505. https://doi.org/10.3390/ph16040505
Urbánek P, Šuly P, Ševčík J, Hanulíková B, Kuřitka I, Šopík T, Stodůlka P. Controlled Drug Delivery Device for Cornea Treatment and Novel Method for Its Testing. Pharmaceuticals. 2023; 16(4):505. https://doi.org/10.3390/ph16040505
Chicago/Turabian StyleUrbánek, Pavel, Pavol Šuly, Jakub Ševčík, Barbora Hanulíková, Ivo Kuřitka, Tomáš Šopík, and Pavel Stodůlka. 2023. "Controlled Drug Delivery Device for Cornea Treatment and Novel Method for Its Testing" Pharmaceuticals 16, no. 4: 505. https://doi.org/10.3390/ph16040505