Pharmacological Profile of the Purinergic P2Y Receptors That Modulate, in Response to ADPβS, the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats
Abstract
:1. Introduction
2. Results
2.1. Systemic Haemodynamic Variables
2.2. Effect of Vehicle or ADPβS Infusions on the Vasodepressor Responses by Electrical Sensory Stimulation or Exogenous α-CGRP
2.3. Effects of Vehicle, MRS2500, PSB0739 or MRS2211 on the Neurogenic Vasodepressor CGRPergic Responses by Electrical Stimulation
2.4. Effect of Vehicle, MRS2500, PSB0739 or MRS2211 on the ADPβS-Induced Inhibition of the Neurogenic Vasodepressor CGRPergic Responses by Electrical Stimulation
2.5. Effect of VEHICLE or glibenclamide on ADPβS-Induced inhibition of the Vasodepressor CGRPergic Responses by Electrical Stimulation
3. Discussion
3.1. General
3.2. Systemic Haemodynamic Variables
3.3. Effect of ADPβS on the Vasodepressor Sensory CGRPergic Drive
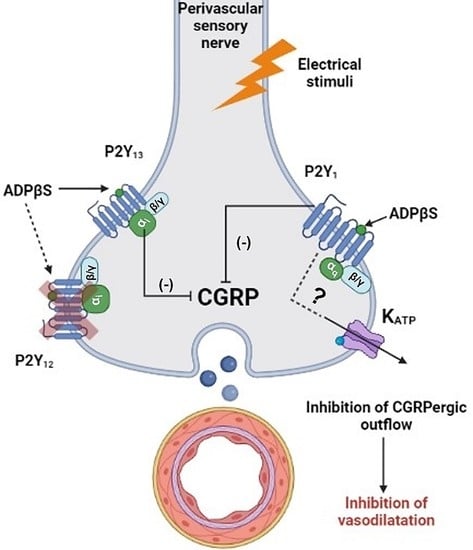
3.4. Inhibition of the Vasodepressor Sensory CGRPergic Drive by ADPβS: Possible Pharmacological Correlation with the Purinergic P2Y1, P2Y12 and P2Y13 Receptor Subtypes
3.5. Are KATP Channels Involved in the Inhibition of the Vasodepressor CGRPergic Drive by ADPβS?
3.6. Limitations of the Study
3.7. Perspectives and Potential Clinical Significance
4. Materials and Methods
4.1. Ethical Approval of the Study Protocol in Pithed Rats
4.2. General Methods
4.3. Experimental Protocols
4.3.1. Protocol I: Selective Electrical Stimulation of the Vasodepressor Sensory CGRPergic Drive
4.3.2. Protocol II: Intravenous Bolus Injections of Exogenous α-CGRP
4.4. Supplementary Procedures
4.5. Compounds
4.6. Data Presentation and Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ADPβS | Adenosine 5′-O-2-thiodiphosphate |
| ATP | Adenosine triphosphate |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| CGRP | Calcitonin gene-related peptide |
| CGRPR | Calcitonin gene-related peptide receptor |
| CLR | Calcitonin-like receptor |
| DBP | Diastolic blood pressure |
| D-R curves | Dose-response curves |
| Ecto-NTPDase 2,3,8 | Ecto-nucleoside triphosphate diphosphohydrolase |
| eNOS | Endothelial nitric oxide synthase |
| GPCR | G protein-coupled receptor |
| NO | Nitric oxide |
| PKA | Protein kinase A |
| RAMP1 | Receptor activity modifying protein |
| S-R curves | Stimulus-response curves |
References
- Amara, S.G.; Jonas, V.; Rosenfeld, M.G.; Ong, E.S.; Evans, R.M. Alternative RNA Processing in Calcitonin Gene Expression Generates MRNAs Encoding Different Polypeptide Products. Nature 1982, 298, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.R.; Panico, M.; Etienne, T.; Tippins, J.; Girgis, S.I.; MacIntyre, I. Isolation and Characterization of Human Calcitonin Gene-Related Peptide. Nature 1984, 308, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin Gene-Related Peptide Is a Potent Vasodilator. Nature 1985, 313, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kawasaki, H.; Imamura, T.; Takasaki, K. Endogenous Calcitonin Gene-Related Peptide Mediates Nonadrenergic Noncholinergic Depressor Response to Spinal Cord Stimulation in the Pithed Rat. Circ. Res. 1992, 71, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, S.J.; Polak, J.M.; Bloom, S.R.; Sabate, I.M.; Mulderry, P.M.; Ghatei, M.A.; Mcgregor, G.P.; Morrison, J.F.B.; Kelly, J.S.; Evans, R.M.; et al. Calcitonin Gene-Related Peptide Immunoreactivity in the Spinal Cord of Man and of Eight Other Species. J. Neurosci. 1984, 4, 3101–3111. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive Peptide Release in the Extracerebral Circulation of Humans during Migraine Headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of Vasoactive Peptides in the Extracerebral Circulation of Humans and the Cat during Activation of the Trigeminovascular System. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef]
- Flühmann, B.; Muff, R.; Hunziker, W.; Fischer, J.A.; Born, W. A Human Orphan Calcitonin Receptor-like Structure. Biochem. Biophys. Res. Commun. 1995, 206, 341–347. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs Regulate the Transport and Ligand Specificity of the Calcitonin-Receptor-like Receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Gray, D.W.; Marshall, I. Human Alpha-Calcitonin Gene-Related Peptide Stimulates Adenylate Cyclase and Guanylate Cyclase and Relaxes Rat Thoracic Aorta by Releasing Nitric Oxide. Br. J. Pharmacol. 1992, 107, 691–696. [Google Scholar] [CrossRef]
- Hong, K.W.; Yoo, S.E.; Yu, S.S.; Lee, J.Y.; Rhim, B.Y. Pharmacological Coupling and Functional Role for CGRP Receptors in the Vasodilation of Rat Pial Arterioles. Am. J. Physiol. 1996, 270 Pt 2, H317–H323. [Google Scholar] [CrossRef] [PubMed]
- Smillie, S.-J.; King, R.; Kodji, X.; Outzen, E.; Pozsgai, G.; Fernandes, E.; Marshall, N.; de Winter, P.; Heads, R.J.; Dessapt-Baradez, C.; et al. An Ongoing Role of α-Calcitonin Gene-Related Peptide as Part of a Protective Network against Hypertension, Vascular Hypertrophy, and Oxidative Stress. Hypertension 2014, 63, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, M.; Moseley, J.M.; Butera, L.; Dusting, G.J.; MacDonald, P.S.; Martin, T.J. Calcitonin Gene-Related Peptide Stimulates Cyclic AMP Formation in Rat Aortic Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 1985, 132, 88–94. [Google Scholar] [CrossRef]
- Nelson, M.T.; Huang, Y.; Brayden, J.E.; Hescheler, J.; Standen, N.B. Arterial Dilations in Response to Calcitonin Gene-Related Peptide Involve Activation of K+ Channels. Nature 1990, 344, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, A.; Kashiwabara, H.; Shibuya, M.; Tanaka, K.; Masaoka, H.; Katayama, S.; Ishii, J. The Interaction of Calcitonin Gene-Related Peptide with Angiotensin II on Blood Pressure and Renin Release. J. Hypertens. Suppl. 1988, 6, S418–S420. [Google Scholar] [CrossRef]
- Gennari, C.; Fischer, J.A. Cardiovascular Action of Calcitonin Gene-Related Peptide in Humans. Calcif. Tissue Int. 1985, 37, 581–584. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain, S.D.; Geppetti, P. Calcitonin Gene-Related Peptide (CGRP) Mechanisms; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2019; Volume 255. [Google Scholar] [CrossRef]
- Preibisz, J.J. Calcitonin Gene-Related Peptide and Regulation of Human Cardiovascular Homeostasis. Am. J. Hypertens. 1993, 6 Pt 1, 434–450. [Google Scholar] [CrossRef]
- Ando, K.; Pegram, B.L.; Frohlich, E.D. Hemodynamic Effects of Calcitonin Gene-Related Peptide in Spontaneously Hypertensive Rats. Am. J. Physiol. 1990, 258 Pt 2, R425–R429. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.H.; Sørgård, M.; di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Hua, X.Y.; Jinno, S.; Back, S.M.; Tam, E.K.; Yaksh, T.L. Multiple Mechanisms for the Effects of Capsaicin, Bradykinin and Nicotine on CGRP Release from Tracheal Afferent Nerves: Role of Prostaglandins, Sympathetic Nerves and Mast Cells. Neuropharmacology 1994, 33, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, H.; Kawasaki, H.; Tezuka, S.; Nakatsuma, A.; Nawa, H.; Araki, H.; Gomita, Y.; Kurosaki, Y. Adrenergic Nerves Mediate Acetylcholine-Induced Endothelium-Independent Vasodilation in the Rat Mesenteric Resistance Artery. Eur. J. Pharmacol. 2001, 419, 231–242. [Google Scholar] [CrossRef]
- Lewis, C.; Neidhart, S.; Holy, C.; North, R.A.; Buell, G.; Surprenant, A. Coexpression of P2X2 and P2X3 Receptor Subunits Can Account for ATP-Gated Currents in Sensory Neurons. Nature 1995, 377, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Villalón, C.M.; Centurión, D.; Rabelo, G.; de Vries, P.; Saxena, P.R.; Sánchez-López, A. The 5-HT1-like Receptors Mediating Inhibition of Sympathetic Vasopressor Outflow in the Pithed Rat: Operational Correlation with the 5-HT1A, 5-HT1B and 5-HT1D Subtypes. Br. J. Pharmacol. 1998, 124, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Hernández, A.; Manrique-Maldonado, G.; Lozano-Cuenca, J.; Muñoz-Islas, E.; Centurión, D.; Maassen VanDenBrink, A.; Villalón, C.M. The 5-HT (1) Receptors Inhibiting the Rat Vasodepressor Sensory CGRPergic Outflow: Further Involvement of 5-HT(1F), but Not 5-HT(1A) or 5-HT(1D), Subtypes. Eur. J. Pharmacol. 2011, 659, 233–243. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.; Muñoz-Islas, E.; Lozano-Cuenca, J.; Ramírez-Rosas, M.B.; Sánchez-López, A.; Centurión, D.; Juan, E.R.S.; Villalón, C.M. Activation of 5-HT1B Receptors Inhibits the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats. Eur. J. Pharmacol. 2010, 637, 131–137. [Google Scholar] [CrossRef]
- Manrique-Maldonado, G.; Altamirano-Espinoza, A.H.; Rivera-Mancilla, E.; Hernández-Abreu, O.; Villalón, C.M. Activation of Dopamine D (3) Receptor Subtypes Inhibits the Neurogenic Systemic Vasodilation Induced by Stimulation of the Perivascular CGRPergic Discharge. ACS Chem. Neurosci. 2019, 10, 3751–3757. [Google Scholar] [CrossRef]
- González-Hernández, A.; Lozano-Cuenca, J.; Marichal-Cancino, B.A.; MaassenVanDenBrink, A.; Villalón, C.M. Dihydroergotamine Inhibits the Vasodepressor Sensory CGRPergic Outflow by Prejunctional Activation of A2-Adrenoceptors and 5-HT1 Receptors. J. Headache Pain 2018, 19, 40. [Google Scholar] [CrossRef] [Green Version]
- Manrique-Maldonado, G.; Altamirano-Espinoza, A.H.; Marichal-Cancino, B.A.; Rivera-Mancilla, E.; Avilés-Rosas, V.; Villalón, C.M. Pharmacological Evidence That Histamine H3 Receptors Inhibit the Vasodepressor Responses by Selective Stimulation of the Rat Perivascular Sensory CGRPergic Outflow. Eur. J. Pharmacol. 2015, 754, 25–31. [Google Scholar] [CrossRef]
- Dunn, P.M.; Liu, M.; Zhong, Y.; King, B.F.; Burnstock, G. Diinosine Pentaphosphate: An Antagonist Which Discriminates between Recombinant P2X(3) and P2X(2/3) Receptors and between Two P2X Receptors in Rat Sensory Neurones. Br. J. Pharmacol. 2000, 130, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Holton, P. The Liberation of Adenosine Triphosphate on Antidromic Stimulation of Sensory Nerves. J. Physiol. 1959, 145, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Haanes, K.A.; Labastida-Ramírez, A.; Blixt, F.W.; Rubio-Beltrán, E.; Dirven, C.M.; Danser, A.H.; Edvinsson, L.; MaassenVanDenBrink, A. Exploration of Purinergic Receptors as Potential Anti-Migraine Targets Using Established Pre-Clinical Migraine Models. Cephalalgia 2019, 39, 1421–1434. [Google Scholar] [CrossRef]
- Chitrakar, I.; Kim-Holzapfel, D.M.; Zhou, W.; French, J.B. Higher Order Structures in Purine and Pyrimidine Metabolism. J. Struct. Biol. 2017, 197, 354–364. [Google Scholar] [CrossRef]
- Gordon, D.B.; Hesse, D.H. Blood Pressure Lowering Action of Adenosine Diphosphate and Related Compounds. Am. J. Physiol. 1961, 201, 1123–1125. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Castillo, B.; Rivera-Mancilla, E.; Haanes, K.A.; MaassenVanDenBrink, A.; Villalón, C.M. The Role of Purinergic P2Y12 and P2Y13 Receptors in ADPβS-Induced Inhibition of the Cardioaccelerator Sympathetic Drive in Pithed Rats. Purinergic Signal. 2020, 16, 73–84. [Google Scholar] [CrossRef] [PubMed]
- von Kügelgen, I. Pharmacological Profiles of Cloned Mammalian P2Y-Receptor Subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.; Yarova, P.; Winter, P.; Dora, K. Desensitization of Endothelial P2Y1 Receptors by PKC-Dependent Mechanisms in Pressurized Rat Small Mesenteric Arteries. Br. J. Pharmacol. 2009, 158, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Zizzo, M.G.; Mulè, F.; Serio, R. Activation of P2Y Receptors by ATP and by Its Analogue, ADPbetaS, Triggers Two Calcium Signal Pathways in the Longitudinal Muscle of Mouse Distal Colon. Eur. J. Pharmacol. 2008, 595, 84–89. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Nerves. Pharmacol. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Burnstock, G. Introduction to Purinergic Signaling. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purinergic Signalling and Neurological Diseases: An Update. CNS Neurol. Disord. Drug Targets 2017, 16, 257–265. [Google Scholar] [CrossRef]
- Burnstock, G.; Ralevic, V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacol. Rev. 2014, 66, 102–192. [Google Scholar] [CrossRef] [PubMed]
- Raqeeb, A.; Sheng, J.; Ao, N.; Braun, A.P. Purinergic P2Y2 Receptors Mediate Rapid Ca(2+) Mobilization, Membrane Hyperpolarization and Nitric Oxide Production in Human Vascular Endothelial Cells. Cell Calcium 2011, 49, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 6855. [Google Scholar] [CrossRef] [PubMed]
- Malmsjö, M.; Erlinge, D.; Högestätt, E.D.; Zygmunt, P.M. Endothelial P2Y Receptors Induce Hyperpolarisation of Vascular Smooth Muscle by Release of Endothelium-Derived Hyperpolarising Factor. Eur. J. Pharmacol. 1999, 364, 169–173. [Google Scholar] [CrossRef]
- Shalev, M.; Staerman, F.; Allain, H.; Lobel, B.; Saïag, B. Stimulation of P2Y Purinoceptors Induces, via Nitric Oxide Production, Endothelium-Dependent Relaxation of Human Isolated Corpus Cavernosum. J. Urol. 1999, 161, 955–959. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and Pyrimidine Receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, J.S.; Maclaren, A.; Pollock, D. A Method of Stimulating Different Segments of the Autonomic Outflow from the Spinal Column to Various Organs in the Pithed Cat and Rat. Br. J. Pharmacol. 1970, 40, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labastida-Ramírez, A.; Rubio-Beltrán, E.; Hernández-Abreu, O.; Daugherty, B.L.; MaassenVanDenBrink, A.; Villalón, C.M. Pharmacological Analysis of the Increases in Heart Rate and Diastolic Blood Pressure Produced by (S)-Isometheptene and (R)-Isometheptene in Pithed Rats. J. Headache Pain 2017, 18, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, C.; García-Pedraza, J.Á.; García, M.; Villalón, C.M.; Morán, A. Role of 5-HT7 Receptors in the Inhibition of the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats. Vasc. Pharmacol. 2014, 63, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Villalón, C.M.; Albarrán-Juárez, J.A.; Lozano-Cuenca, J.; Pertz, H.H.; Görnemann, T.; Centurión, D. Pharmacological Profile of the Clonidine-Induced Inhibition of Vasodepressor Sensory Outflow in Pithed Rats: Correlation with Alpha(2A/2C)-Adrenoceptors. Br. J. Pharmacol. 2008, 154, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Hernández, A.; Marichal-Cancino, B.A.; Lozano-Cuenca, J.; MaassenVanDenBrink, A.; Villalón, C.M. Functional Characterization of the Prejunctional Receptors Mediating the Inhibition by Ergotamine of the Rat Perivascular Sensory Peptidergic Drive. ACS Chem. Neurosci. 2019, 10, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Villalón, C.M.; Contreras, J.; Ramírez-San Juan, E.; Castillo, C.; Perusquía, M.; López-Muñoz, F.J.; Terrón, J.A. 5-Hydroxytryptamine Inhibits Pressor Responses to Preganglionic Sympathetic Nerve Stimulation in Pithed Rats. Life Sci. 1995, 57, 803–812. [Google Scholar] [CrossRef]
- Avilés-Rosas, V.H.; Rivera-Mancilla, E.; Marichal-Cancino, B.A.; Manrique-Maldonado, G.; Altamirano-Espinoza, A.H.; Maassen Van Den Brink, A.; Villalón, C.M. Olcegepant Blocks Neurogenic and Non-Neurogenic CGRPergic Vasodepressor Responses and Facilitates Noradrenergic Vasopressor Responses in Pithed Rats. Br. J. Pharmacol. 2017, 174, 2001–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saïag, B.; Hillaire-Buys, D.; Chapal, J.; Petit, P.; Pape, D.; Rault, B.; Allain, H.; Loubatières-Mariani, M.M. Study of the Mechanisms Involved in Adenosine-5′-O-(2-Thiodiphosphate) Induced Relaxation of Rat Thoracic Aorta and Pancreatic Vascular Bed. Br. J. Pharmacol. 1996, 118, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for Purines and Pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar]
- Bennet, D.W.; Drury, A.N. Further Observations Relating to the Physiological Activity of Adenine Compounds. J. Physiol. 1931, 72, 288–320. [Google Scholar] [CrossRef]
- Wihlborg, A.-K.; Malmsjö, M.; Eyjolfsson, A.; Gustafsson, R.; Jacobson, K.; Erlinge, D. Extracellular Nucleotides Induce Vasodilatation in Human Arteries via Prostaglandins, Nitric Oxide and Endothelium-Derived Hyperpolarising Factor. Br. J. Pharmacol. 2003, 138, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Drury, A.N.; Szent-Györgyi, A. The Physiological Activity of Adenine Compounds with Especial Reference to Their Action upon the Mammalian Heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.; Baghdadi, A. Functional Role of Adenosine via KATP Channels in Cerebral Capillary Endothelial Cells and Pericytes. Purinergic Signal. 2022, 18, 249–251. [Google Scholar] [CrossRef]
- Zhang, F.L.; Luo, L.; Gustafson, E.; Palmer, K.; Qiao, X.; Fan, X.; Yang, S.; Laz, T.M.; Bayne, M.; Monsma, F. P2Y13: Identification and Characterization of a Novel Gαi-Coupled ADP Receptor from Human and Mouse. J. Pharmacol. Exp. Ther. 2002, 301, 705–713. [Google Scholar] [CrossRef]
- Zhang, F.L.; Luo, L.; Gustafson, E.; Lachowicz, J.; Smith, M.; Qiao, X.; Liu, Y.-H.; Chen, G.; Pramanik, B.; Laz, T.M.; et al. ADP Is the Cognate Ligand for the Orphan G Protein-Coupled Receptor SP1999. J. Biol. Chem. 2001, 276, 8608–8615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldo, G.L.; Harden, T.K. Agonist Binding and Gq-Stimulating Activities of the Purified Human P2Y1 Receptor. Mol. Pharmacol. 2004, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricks, I.P.; Carter, R.L.; Lazarowski, E.R.; Harden, T.K. Gi -Dependent Cell Signaling Responses of the Human P2Y14 Receptor in Model Cell Systems. J. Pharmacol. Exp. Ther. 2009, 330, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baqi, Y.; Atzler, K.; Köse, M.; Glänzel, M.; Müller, C.E. High-Affinity, Non-Nucleotide-Derived Competitive Antagonists of Platelet P2Y12 Receptors. J. Med. Chem. 2009, 52, 3784–3793. [Google Scholar] [CrossRef]
- Hoffmann, K.; Baqi, Y.; Morena, M.S.; Glänzel, M.; Müller, C.E.; von Kügelgen, I. Interaction of New, Very Potent Non-Nucleotide Antagonists with Arg256 of the Human Platelet P2Y12 Receptor. J. Pharmacol. Exp. Ther. 2009, 331, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Ohno, M.; Xu, B.; Kim, H.O.; Choi, Y.; Ji, X.D.; Maddileti, S.; Marquez, V.E.; Harden, T.K.; Jacobson, K. A. 2-Substitution of Adenine Nucleotide Analogues Containing a Bicyclo[3.1.0]Hexane Ring System Locked in a Northern Conformation: Enhanced Potency as P2Y1 Receptor Antagonists. J. Med. Chem. 2003, 46, 4974–4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-C.; Lee, J.-S.; Sak, K.; Marteau, F.; Mamedova, L.; Boeynaems, J.-M.; Jacobson, K.A. Synthesis of Pyridoxal Phosphate Derivatives with Antagonist Activity at the P2Y13 Receptor. Biochem. Pharmacol. 2005, 70, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, M.; Lecchi, A.; Ohno, M.; Joshi, B.v.; Besada, P.; Tchilibon, S.; Lombardi, R.; Bischofberger, N.; Harden, T.K.; Jacobson, K.A. Antiaggregatory Activity in Human Platelets of Potent Antagonists of the P2Y1 Receptor. Biochem. Pharmacol. 2004, 68, 1995–2002. [Google Scholar] [CrossRef] [Green Version]
- Marteau, F.; le Poul, E.; Communi, D.; Communi, D.; Labouret, C.; Savi, P.; Boeynaems, J.-M.; Gonzalez, N.S. Pharmacological Characterization of the Human P2Y13 Receptor. Mol. Pharmacol. 2003, 64, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Sen, R.; Gómez-Villafuertes, R.; Ortega, F.; Gualix, J.; Delicado, E.G.; Miras-Portugal, M.T. An Update on P2Y13 Receptor Signalling and Function. Adv. Exp. Med. Biol. 2017, 1051, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, J.F.; Cinalli, A.R.; Fernández, V.; Roquel, L.I.; Losavio, A.S. P2Y13 Receptors Mediate Presynaptic Inhibition of Acetylcholine Release Induced by Adenine Nucleotides at the Mouse Neuromuscular Junction. Neuroscience 2016, 326, 31–44. [Google Scholar] [CrossRef]
- Malin, S.A.; Molliver, D.C. Gi- and Gq-Coupled ADP (P2Y) Receptors Act in Opposition to Modulate Nociceptive Signaling and Inflammatory Pain Behavior. Mol. Pain 2010, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H. Ectonucleoside Triphosphate Diphosphohydrolases and Ecto-5′-Nucleotidase in Purinergic Signaling: How the Field Developed and Where We Are Now. In Purinergic Signalling; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2021; pp. 117–125. [Google Scholar] [CrossRef]
- Quayle, J.M.; Standen, N.B. KATP Channels in Vascular Smooth Muscle. Cardiovasc. Res. 1994, 28, 797–804. [Google Scholar] [CrossRef]
- Brayden, J.E. Functional Roles of KATP Channels in Vascular Smooth Muscle. Clin. Exp. Pharmacol. Physiol. 2002, 29, 312–316. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef] [Green Version]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium Channels in Vascular Smooth Muscle: A Pathophysiological and Pharmacological Perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Simon, J.; Filippov, A.K.; Göransson, S.; Wong, Y.H.; Frelin, C.; Michel, A.D.; Brown, D.A.; Barnard, E.A. Characterization and Channel Coupling of the P2Y12 Nucleotide Receptor of Brain Capillary Endothelial Cells. J. Biol. Chem. 2002, 277, 31390–31400. [Google Scholar] [CrossRef] [Green Version]
- Schicker, K.W.; Chandaka, G.K.; Geier, P.; Kubista, H.; Boehm, S. P2Y1 Receptors Mediate an Activation of Neuronal Calcium-Dependent K+ Channels. J. Physiol. 2010, 588 Pt 19, 3713–3725. [Google Scholar] [CrossRef]
- Dsouza, C.; Komarova, S.V. Characterization of Potency of the P2Y13 Receptor Agonists: A Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 3468. [Google Scholar] [CrossRef] [PubMed]
- Traserra, S.; Barber, C.; Maclnnes, J.; Relea, L.; MacPherson, L.C.; Cunningham, M.R.; Vergara, P.; Accarino, A.; Kennedy, C.; Jimenez, M. Different Responses of the Blockade of the P2Y1 Receptor with BPTU in Human and Porcine Intestinal Tissues and in Cell Cultures. Neurogastroenterol. Motil. 2021, 33, e14101. [Google Scholar] [CrossRef]
- Van Giezen, J.J.J.; Sidaway, J.; Glaves, P.; Kirk, I.; Björkman, J.A. Ticagrelor Inhibits Adenosine Uptake in Vitro and Enhances Adenosine-Mediated Hyperemia Responses in a Canine Model. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Potts, J.D.; DiPette, D.J. Protective Role of α-Calcitonin Gene-Related Peptide in Cardiovascular Diseases. Front. Physiol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Rivera-Mancilla, E.; Villalón, C.M.; MaassenVanDenBrink, A. CGRP Inhibitors for Migraine Prophylaxis: A Safety Review. Expert Opin. Drug Saf. 2020, 19, 1237–1250. [Google Scholar] [CrossRef]
- NOM-062-ZOO-1999; Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: Mexico City, Mexico, 1999.
- Bayne, K. Revised Guide for the Care and Use of Laboratory Animals Available. American Physiological Society. Physiologist 1996, 39, 208–211. [Google Scholar]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for Reporting Experiments Involving Animals: The ARRIVE Guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biomedical Approach, 2nd ed.; McGraw-Hill: Kogakusha Ltd.: Tokyo, Japan, 1980. [Google Scholar]






| Treatment | Doses | DBP (mm Hg) | ||
|---|---|---|---|---|
| Before | After 10 min | % Change DBP | ||
| Control (no treatment) | 108 ± 4 | − | 0 ± 0 | |
| Bidistilled water | 1 mL/kg a | 108 ± 4 | 113 ± 4 | 4 ± 3 |
| Bidistilled water | 0.02 mL/min b | 103 ± 2 | 103 ± 3 | 1 ± 1 |
| ADPβS (adenosine 5′-O-2- thiodiphosphate) | 3 µg/kg·min b | 116 ± 5 | 110 ± 5 | −5 ± 2 |
| 5.6 µg/kg·min b | 111 ± 3 | 90 ± 5 Δ | −19 ± 3 Δ◊□ | |
| 10 µg/kg·min b | 105 ± 3 | 73 ± 3 Δ | −30 ± 5 Δ◊□○ | |
| MRS2500 | 300 µg/kg a | 110 ± 3 | 111 ± 3 | 2 ± 3 |
| MRS2500 + ADPβS | 300 µg/kg a + 5.6 µg/kg·min b | 114 ± 6 | 114 ± 5 | 0 ± 1 |
| PSB0739 | 300 µg/kg a | 106 ± 4 | 109 ± 4 | 2 ± 2 |
| PSB0739 + ADPβS | 300 µg/kg a + 5.6 µg/kg·min b | 116 ± 4 | 97 ± 5 | −17 ± 2 Δ◊□ |
| MRS2211 | 1000 µg/kga | 109 + 3 | 109 ± 3 | 0 ± 0 |
| MRS2211 + ADPβS | 1000 µg/kga + 5.6 µg/kg·minb | 114 ± 4 | 94 ± 5 | −18 ± 3 Δ◊□ |
| MRS2211 | 3000 µg/kga | 110 ± 2 | 110 ± 2 | 0 ± 0 |
| MRS2211 + ADPβS | 3000 µg/kg a + 5.6 µg/kg·min b | 120 ± 3 | 103 ± 3 | −13 ± 2 Δ◊□ |
| Vehicle of glibenclamide | 1 mL/kg a | 107 ± 4 | 106 ± 5 | −1 ± 2 |
| Vehicle of glibenclamide + ADPβS | 1 mL/kg a + 5.6 µg/kg·min b | 106 ± 3 | 106 ± 3 | 0 ± 0 |
| Glibenclamide | 20 mg/kg a | 108 ± 2 | 108 ± 2 | 0 ± 0 |
| Glibenclamide + ADPβS | 20 mg/kg a + 5.6 µg/kg·min b | 103 ± 1 | 102 ± 2 | −1 ± 2 |
| Group | 0.56 Hz | 1.0 Hz | 1.8 Hz | 3.1 Hz | 5.6 Hz |
|---|---|---|---|---|---|
| Control | −4.0 ± 0.3 | −8.5 ± 0.9 | −14.0 ± 1.6 | −23.8 ± 2.1 | −32.6 ± 2.5 |
| Vehicle (0.02 mL/min) | −4.9 ± 1.3 | −10.7 ± 1.3 | −16.7 ± 1.4 | −24.4 ± 2.7 | −30.4 ± 2.4 |
| ADPβS (3 µg/kg·min) | −3.5 ± 0.4 | −9.2 ± 1.7 | −14.5 ± 1.5 | −23.9 ± 2.3 | −31.2 ± 2.8 |
| ADPβS (5.6 µg/kg·min) | −2.4 ± 0.7 | −4.1 ± 0.5 | −6.4 ± 0.6 Δ◊□ | −12.2 ± 0.9 Δ◊□ | −19.1 ± 1.0 Δ◊□ |
| ADPβS (10 µg/kg·min) | −1.1 ± 1.1 | −4.1 ± 1.3 | −8.2 ± 1.1 Δ◊□ | −14.3 ± 1.2 Δ◊□ | −20.4 ± 1.2 Δ◊□ |
| Receptors | P2Y1 | P2Y12 | P2Y13 | |
|---|---|---|---|---|
| Drugs | ||||
| Agonist | ADPβS | 5.6 | 7.5 | 7.5 |
| Antagonists | MRS2500 | 9.1 | 4 a | 4 a |
| PSB0739 | 6 b | 7.6 | 6 b | |
| MRS2211 | 5 c | 5 c | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel-Martínez, A.D.; Linares-Bedolla, J.; Villanueva-Castillo, B.; Haanes, K.A.; MaassenVanDenBrink, A.; Villalón, C.M. Pharmacological Profile of the Purinergic P2Y Receptors That Modulate, in Response to ADPβS, the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats. Pharmaceuticals 2023, 16, 475. https://doi.org/10.3390/ph16030475
Miguel-Martínez AD, Linares-Bedolla J, Villanueva-Castillo B, Haanes KA, MaassenVanDenBrink A, Villalón CM. Pharmacological Profile of the Purinergic P2Y Receptors That Modulate, in Response to ADPβS, the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats. Pharmaceuticals. 2023; 16(3):475. https://doi.org/10.3390/ph16030475
Chicago/Turabian StyleMiguel-Martínez, Alejandro D., Juan Linares-Bedolla, Belinda Villanueva-Castillo, Kristian A. Haanes, Antoinette MaassenVanDenBrink, and Carlos M. Villalón. 2023. "Pharmacological Profile of the Purinergic P2Y Receptors That Modulate, in Response to ADPβS, the Vasodepressor Sensory CGRPergic Outflow in Pithed Rats" Pharmaceuticals 16, no. 3: 475. https://doi.org/10.3390/ph16030475