Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research
Abstract
:1. Introduction
2. Methodology
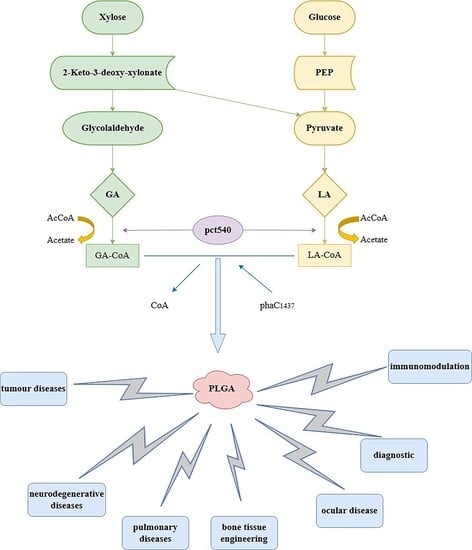
3. The Synthesis of PLGA
4. The Physicochemical Properties of PLGA
5. The Degradation Properties of PLGA
6. The Applications of PLGA to Biomedical Research
6.1. In-Tumor Diseases
6.2. In Neurodegenerative Diseases
6.3. In Pulmonary Diseases
6.4. In Bone Tissue Engineering
6.5. In Ocular Disease
6.6. In Diagnostic
6.7. In Immunomodulation
6.8. In Inflammatory Diseases
6.9. In Cardiovascular Diseases
6.10. In Infection
7. Prospectives and Research Gaps
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Y.; Song, X.; Zhang, Z.; Zhuang, X.; He, C.; Chen, X. Biodegradable, pH-Responsive Carboxymethyl Cellulose/Poly(Acrylic Acid) Hydrogels for Oral Insulin Delivery. Macromol. Biosci. 2014, 14, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Jašo, V.; Glenn, G.; Klamczynski, A.; Petrović, Z.S. Biodegradability study of polylactic acid/ thermoplastic polyurethane blends. Polym. Test. 2015, 47, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Keshavarz Moraveji, M. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef] [Green Version]
- Koerner, J.; Horvath, D.; Groettrup, M. Harnessing Dendritic Cells for Poly (D,L-lactide-co-glycolide) Microspheres (PLGA MS)—Mediated Anti-tumor Therapy. Front. Immunol. 2019, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Moon, J.-H.; Jeong, S.-U.; Jung, H.-H.; Park, C.-S.; Hwang, B.Y.; Lee, C.-K. Induction of antigen-specific immune tolerance using biodegradable nanoparticles containing antigen and dexamethasone. Int. J. Nanomed. 2019, 14, 5229–5242. [Google Scholar] [CrossRef] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, X. Microfluidics for producing poly (lactic-co-glycolic acid)-based pharmaceutical nanoparticles. Adv. Drug Deliv. Rev. 2018, 128, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jain, D.S.; Chakraborty, S. Poly Lactic-Co-Glycolic Acid (PLGA) Copolymer and Its Pharmaceutical Application. Handb. Polym. Pharm. Technol. 2015, 2, 151–172. [Google Scholar]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Health Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Erbetta, C.D.A.C.; Alves, R.J.; Resende, J.M.; Freitas, R.F.d.S.; Geraldo de Sousa, R. Synthesis and Characterization of Poly(D,L-Lactide-co-Glycolide) Copolymer. J. Biomater. Nanobiotechnology 2012, 3, 208–225. [Google Scholar] [CrossRef]
- Kaihara, S.; Matsumura, S.; Mikos, A.G.; Fisher, J.P. Synthesis of poly(L-lactide) and polyglycolide by ring-opening polymerization. Nat. Protoc. 2007, 2, 2767–2771. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Y.; Park, S.J.; Kim, W.J.; Yang, J.E.; Lee, H.; Shin, J.; Lee, S.Y. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat. Biotechnol. 2016, 34, 435–440. [Google Scholar] [CrossRef]
- Lamprecht, A.; Ubrich, N.; Pérez, M.H.; Lehr, C.-M.; Hoffman, M.; Maincent, P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int. J. Pharm. 2000, 196, 177–182. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Mchugh, A.; Graham, P.; Brodbeck, K. Phase Inversion Dynamics of PLGA Solutions Related to Drug Delivery. MRS Proc. 1999, 550, 41–46. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. Part A 2006, 76A, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Park, T.G. Degradation of poly(lactic-co-glycolic acid) microspheres: Effect of copolymer composition. Biomaterials 1995, 16, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Villemin, E.; Ong, Y.C.; Thomas, C.M.; Gasser, G. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat. Rev. Chem. 2019, 3, 261–282. [Google Scholar] [CrossRef]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Park, T.G. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J. Biomed. Mater. Res. 2001, 55, 401–408. [Google Scholar] [CrossRef]
- Siepmann, J.; Elkharraz, K.; Siepmann, F.; Klose, D. How Autocatalysis Accelerates Drug Release from PLGA-Based Microparticles: A Quantitative Treatment. Biomacromolecules 2005, 6, 2312–2319. [Google Scholar] [CrossRef]
- Go, E.J.; Kang, E.Y.; Lee, S.K.; Park, S.; Kim, J.H.; Park, W.; Kim, I.H.; Choi, B.; Han, D.K. An osteoconductive PLGA scaffold with bioactive β-TCP and anti-inflammatory Mg(OH)2 to improve in vivo bone regeneration. Biomater. Sci. 2020, 8, 937–948. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Li, J.; Jiang, G.; Ding, F. The effect of pH on the polymer degradation and drug release from PLGA-mPEG microparticles. J. Appl. Polym. Sci. 2008, 109, 475–482. [Google Scholar] [CrossRef]
- Amann, L.C.; Gandal, M.; Lin, R.; Liang, Y.; Siegel, S.J. In Vitro–In Vivo Correlations of Scalable PLGA-Risperidone Implants for the Treatment of Schizophrenia. Pharm. Res. 2010, 27, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Bao, Q.; Burgess, D.J. In vitro-in vivo correlation of PLGA microspheres: Effect of polymer source variation and temperature. J. Control. Release 2022, 347, 347–355. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [Green Version]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Fong, P.M.; Lu, J.; Russell, K.S.; Booth, C.J.; Saltzman, W.M.; Fahmy, T.M. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2017, 5, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- Le Reste, P.J.; Pineau, R.; Voutetakis, K.; Samal, J.; Jégou, G.; Lhomond, S.; Gorman, A.M.; Samali, A.; Patterson, J.B.; Zeng, Q.; et al. Local intracerebral inhibition of IRE1 by MKC8866 sensitizes glioblastoma to irradiation/chemotherapy in vivo. Cancer Lett. 2020, 494, 73–83. [Google Scholar] [CrossRef]
- Rahn, J.J.; Lun, X.; Jorch, S.K.; Hao, X.; Venugopal, C.; Vora, P.; Ahn, B.Y.; Babes, L.; Alshehri, M.M.; Cairncross, J.G.; et al. Development of a peptide-based delivery platform for targeting malignant brain tumors. Biomaterials 2020, 252, 120105. [Google Scholar] [CrossRef]
- Floyd, J.A.; Galperin, A.; Ratner, B.D. Drug encapsulated polymeric microspheres for intracranial tumor therapy: A review of the literature. Adv. Drug Deliv. Rev. 2015, 91, 23–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.R.; Kim, J.; Schiapparelli, P.; Vazquez-Ramos, C.A.; Martinez-Gutierrez, J.C.; Ruiz-Valls, A.; Inman, K.; Shamul, J.G.; Green, J.J.; Quinones-Hinojosa, A. Verteporfin-Loaded Polymeric Microparticles for Intratumoral Treatment of Brain Cancer. Mol. Pharm. 2019, 16, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Shavi, G.V.; Nayak, U.Y.; Reddy, M.S.; Ginjupalli, K.; Deshpande, P.B.; Averineni, R.K.; Udupa, N.; Sadhu, S.S.; Danilenkoff, C.; Raghavendra, R. A novel long-acting biodegradable depot formulation of anastrozole for breast cancer therapy. Mater. Sci. Eng. C 2017, 75, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chen, W.-B.; Zhang, X.-Y.; Kang, X.-N.; Jin, L.-J.; Zhang, H.; Wang, Z.-Y. HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J. Stem Cells 2020, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-De-La-Cruz, E.; Muñoz, M.D.L.; Pérez-Muñoz, A.; García-Hernández, N.; Meza, C.M.; Hinojosa-Cruz, J.C. Reduced mitochondrial DNA copy number is associated with the haplogroup, and some clinical features of breast cancer in Mexican patients. Gene 2020, 761, 145047. [Google Scholar] [CrossRef] [PubMed]
- Karthick, V.; Panda, S.; Kumar, V.G.; Kumar, D.; Shrestha, L.K.; Ariga, K.; Vasanth, K.; Chinnathambi, S.; Dhas, T.S.; Suganya, K.U. Quercetin loaded PLGA microspheres induce apoptosis in breast cancer cells. Appl. Surf. Sci. 2019, 487, 211–217. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, M.; Hu, X.; Leon Plata, P.A.; Ciupinski, P.; Wang, Z.J.; Liu, Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int. J. Pharm. 2016, 511, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Correction to Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2019, 13, 7355. [Google Scholar] [CrossRef] [Green Version]
- Barcia, E.; Boeva, L.; García-García, L.; Slowing, K.; Fernández-Carballido, A.; Casanova, Y.; Negro, S. Nanotechnology-based drug delivery of ropinirole for Parkinson’s disease. Drug Deliv. 2017, 24, 1112–1123. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.; Khan, A.A.; Mahmood, S. Nose to brain delivery of selegiline loaded PLGA/lipid nanoparticles: Synthesis, characterisation and brain pharmacokinetics evaluation. J. Drug Deliv. Sci. Technol. 2022, 77, 103923. [Google Scholar] [CrossRef]
- Cook, R.O.; Pannu, R.K.; Kellaway, I.W. Novel sustained release microspheres for pulmonary drug delivery. J. Control. Release 2005, 104, 79–90. [Google Scholar] [CrossRef]
- Barnes, P.J. Chronic Obstructive Pulmonary Disease: A Growing but Neglected Global Epidemic. PLoS Med. 2007, 4, e112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, J.; Bisen, M.; Misra, A.; Srivastava, V.K.; Kaushik, S.; Siddiqui, A.J.; Mishra, N.; Singh, A.; Jyoti, A. Targeting COPD with PLGA-Based Nanoparticles: Current Status and Prospects. BioMed Res. Int. 2022, 2022, 5058121. [Google Scholar] [CrossRef] [PubMed]
- Erni, S.T.; Fernandes, G.; Buri, M.; Perny, M.; Rutten, R.J.; Van Noort, J.M.; Senn, P.; Grandgirard, D.; Roccio, M.; Leib, S.L. Anti-inflammatory and Oto-Protective Effect of the Small Heat Shock Protein Alpha B-Crystallin (HspB5) in Experimental Pneumococcal Meningitis. Front. Neurol. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Noort, J.M.; Bsibsi, M.; Nacken, P.J.; Gerritsen, W.H.; Amor, S.; Holtman, I.R.; Boddeke, E.; van Ark, I.; Leusink-Muis, T.; Folkerts, G.; et al. Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials 2013, 34, 831–840. [Google Scholar] [CrossRef]
- Marcianes, P.; Negro, S.; Barcia, E.; Montejo, C.; Fernández-Carballido, A. Potential Active Targeting of Gatifloxacin to Macrophages by Means of Surface-Modified PLGA Microparticles Destined to Treat Tuberculosis. AAPS PharmSciTech 2020, 21, 15. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.R.; Hatt, L.P.; Rosenberg, G.S.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Muscolo, D.L.; Ayerza, M.A.; Aponte-Tinao, L.A. Massive Allograft Use in Orthopedic Oncology. Orthop. Clin. N. Am. 2006, 37, 65–74. [Google Scholar] [CrossRef]
- Sun, F.; Sun, X.; Wang, H.; Li, C.; Zhao, Y.; Tian, J.; Lin, Y. Application of 3D-Printed, PLGA-Based Scaffolds in Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5831. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, C.; Zhang, R.; Wang, X.; Li, X. Applications of Graphene and Its Derivatives in Bone Repair: Advantages for Promoting Bone Formation and Providing Real-Time Detection, Challenges and Future Prospects. Int. J. Nanomed. 2020, 15, 7523–7551. [Google Scholar] [CrossRef] [PubMed]
- Esrafilzadeh, D.; Jalili, R.; Stewart, E.M.; Aboutalebi, S.H.; Razal, J.M.; Moulton, S.E.; Wallace, G.G. High-Performance Multifunctional Graphene-PLGA Fibers: Toward Biomimetic and Conducting 3D Scaffolds. Adv. Funct. Mater. 2016, 26, 3105–3117. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701161. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef]
- Lin, S.; Cui, L.; Chen, G.; Huang, J.; Yang, Y.; Zou, K.; Lai, Y.; Wang, X.; Zou, L.; Wu, T.; et al. PLGA/β-TCP composite scaffold incorporating salvianolic acid B promotes bone fusion by angiogenesis and osteogenesis in a rat spinal fusion model. Biomaterials 2019, 196, 109–121. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X.; Li, H.; Lu, H.; Oswald, J.; Liu, Y.; Zeng, J.; Jin, C.; Peng, X.; Liu, J.; et al. iRGD decorated liposomes: A novel actively penetrating topical ocular drug delivery strategy. Nano Res. 2020, 13, 3105–3109. [Google Scholar] [CrossRef]
- Bravo-Osuna, I.; Andrés-Guerrero, V.; Arranz-Romera, A.; Pérez, S.E.; Molina-Martínez, I.T.; Herrero-Vanrell, R. Microspheres as intraocular therapeutic tools in chronic diseases of the optic nerve and retina. Adv. Drug Deliv. Rev. 2018, 126, 127–144. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Otsuka, T.; Naito, A.; Shimazawa, M.; Hara, H. Triamcinolone Acetonide Suppresses Inflammation and Facilitates Vascular Barrier Function in Human Retinal Microvascular Endothelial Cells. Curr. Neurovascular Res. 2017, 14, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Balgemann, R.; Greb, C.; Nunn, B.M.; Ueda, S.; Noma, H.; McDonald, K.; Kaplan, H.J.; Tamiya, S.; O’Toole, M.G. Production of dasatinib encapsulated spray-dried poly (lactic-co-glycolic acid) particles. J. Drug Deliv. Sci. Technol. 2019, 53, 101204. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Vega, E.; Pérez, Y.; Gómara, M.J.; García, M.L.; Haro, I. Conjugation of cell-penetrating peptides with poly(lactic-co-glycolic acid)-polyethylene glycol nanoparticles improves ocular drug delivery. Int. J. Nanomed. 2015, 10, 609–631. [Google Scholar] [CrossRef] [Green Version]
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Li, T.; Chen, Z.; Geng, Y.; Xie, X.; Li, S.; Yang, H.; Wu, C.; Liu, Y. Luminescent/magnetic PLGA-based hybrid nanocomposites: A smart nanocarrier system for targeted codelivery and dual-modality imaging in cancer theranostics. Int. J. Nanomed. 2017, 12, 4299–4322. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef]
- Niu, C.; Wang, Z.; Lu, G.; Krupka, T.M.; Sun, Y.; You, Y.; Song, W.; Ran, H.; Li, P.; Zheng, Y. Doxorubicin loaded superparamagnetic PLGA-iron oxide multifunctional microbubbles for dual-mode US/MR imaging and therapy of metastasis in lymph nodes. Biomaterials 2013, 34, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Chen, Z.; Xie, X.; Zhang, H.; Feng, Y.; Li, S.; Qin, X.; Yang, H.; Wu, C.; et al. NIR-Light-Triggered Anticancer Strategy for Dual-Modality Imaging-Guided Combination Therapy via a Bioinspired Hybrid PLGA Nanoplatform. Mol. Pharm. 2019, 16, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Bennewitz, M.F.; Lobo, T.L.; Nkansah, M.K.; Ulas, G.; Brudvig, G.W.; Shapiro, E.M. Biocompatible and pH-Sensitive PLGA Encapsulated MnO Nanocrystals for Molecular and Cellular MRI. ACS Nano 2011, 5, 3438–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Cheng, X.; Jiang, J.; Li, T.; Zhang, Z.; Tsauo, C.; Liu, Y.; Wang, Z. Dual-modal photoacoustic and magnetic resonance tracking of tendon stem cells with PLGA/iron oxide microparticles in vitro. PLoS ONE 2018, 13, e0193362/0193361. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Dark, J.H. Organ transplantation: Historical perspective and current practice. Br. J. Anaesth. 2012, 108 (Suppl. 1), i29–i42. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.S.; Madsen, J.C. Recent advances in the immunology of chronic rejection. Curr. Opin. Nephrol. Hypertens. 2002, 11, 315–321. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, A.M.J.S.M.P.C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Coronel, M.; Liang, J.-P.; Li, Y.; Stabler, C. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials 2019, 210, 1–11. [Google Scholar] [CrossRef]
- Li, Y.; Frei, A.W.; Labrada, I.M.; Rong, Y.; Liang, J.-P.; Samojlik, M.M.; Sun, C.; Barash, S.; Keselowsky, B.G.; Bayer, A.L.; et al. Immunosuppressive PLGA TGF-β1 Microparticles Induce Polyclonal and Antigen-Specific Regulatory T Cells for Local Immunomodulation of Allogeneic Islet Transplants. Front. Immunol. 2021, 12, 653088. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, L.; Pandeya, A.; Cui, J.; Zhang, Y.; Li, Z. Pyroptosis-Induced Inflammation and Tissue Damage. J. Mol. Biol. 2022, 434, 167301. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-M.; Yang, Y.-J.; Zhang, L.; Zhang, X.; Guan, F.-F.; Zhang, L.-F. Naringin Enhances CaMKII Activity and Improves Long-Term Memory in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2013, 14, 5576–5586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Cheng, W.; Qin, Z.; Yu, H.; Yu, Z.; Zhong, M.; Sun, K.; Zhang, W. Effects of Naringin on Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells In Vitro and In Vivo. Stem Cells Int. 2015, 2015, 758706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.; Konkimalla, V.B.; Pal, A.; Sharma, T.; Si, S.C. Naringin as Sustained Delivery Nanoparticles Ameliorates the Anti-inflammatory Activity in a Freund’s Complete Adjuvant-Induced Arthritis Model. ACS Omega 2021, 6, 28630–28641. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Esteruelas, G.; Ortiz, A.; Espina, M.; Prat, J.; Muñoz, M.; Cano, A.; Calpena, A.C.; Ettcheto, M.; Camins, A.; et al. Dexibuprofen Biodegradable Nanoparticles: One Step Closer towards a Better Ocular Interaction Study. Nanomaterials 2020, 10, 720. [Google Scholar] [CrossRef] [Green Version]
- Galindo, R.; Sánchez-López, E.; Gómara, M.J.; Espina, M.; Ettcheto, M.; Cano, A.; Haro, I.; Camins, A.; García, M.L. Development of Peptide Targeted PLGA-PEGylated Nanoparticles Loading Licochalcone-A for Ocular Inflammation. Pharmaceutics 2022, 14, 285. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Wright, F.L.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Ortega-Rivera, O.A.; Shin, M.D.; Moreno-Gonzalez, M.A.; Pokorski, J.K.; Steinmetz, N.F. A Single-Dose Qβ VLP Vaccine against S100A9 Protein Reduces Atherosclerosis in a Preclinical Model. Adv. Ther. 2022, 5, 2200092. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Ju, R.; Chen, K.; Li, S.; Wang, W.; Yan, Y. In vivo biocompatibility and hemocompatibility of a polytetrafluoroethylene small diameter vascular graft modified with sulfonated silk fibroin. Am. J. Surg. 2017, 213, 87–93. [Google Scholar] [CrossRef]
- Hasanpour, A.; Esmaeili, F.; Hosseini, H.; Amani, A. Use of mPEG-PLGA nanoparticles to improve bioactivity and hemocompatibility of streptokinase: In-vitro and in-vivo studies. Mater. Sci. Eng. C 2021, 118, 111427. [Google Scholar] [CrossRef]
- Zamanlu, M.; Eskandani, M.; Barar, J.; Jaymand, M.; Pakchin, P.S.; Farhoudi, M. Enhanced thrombolysis using tissue plasminogen activator (tPA)-loaded PEGylated PLGA nanoparticles for ischemic stroke. J. Drug Deliv. Sci. Technol. 2019, 53, 101165. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. Antibiotic-Resistant Bugs in the 21st Century—A Clinical Super-Challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies To Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Douros, A.; Grabowski, K.; Stahlmann, R. Drug–drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Raza, F.; Wang, X.; Zhang, S.; Rong, R.; Qiu, M.; Su, J. Red Blood Cell Membrane-Camouflaged Tedizolid Phosphate-Loaded PLGA Nanoparticles for Bacterial-Infection Therapy. Pharmaceutics 2021, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Beck-Broichsitter, M.; Banga, A.K. Design and Evaluation of a Poly(Lactide-co-Glycolide)-Based In Situ Film-Forming System for Topical Delivery of Trolamine Salicylate. Pharmaceutics 2019, 11, 409. [Google Scholar] [CrossRef] [Green Version]
- Šnejdrová, E.; Martiška, J.; Loskot, J.; Paraskevopoulos, G.; Kováčik, A.G.R., Jr.; Budai-Szűcs, M.; Palát, K.; Konečná, K. PLGA based film forming systems for superficial fungal infections treatment. Eur. J. Pharm. Sci. 2021, 163, 105855. [Google Scholar] [CrossRef]
- Yan, J.; Fei, W.; Song, Q.; Zhu, Y.; Bu, N.; Wang, L.; Zhao, M.; Zheng, X. Cell membrane-camouflaged PLGA biomimetic system for diverse biomedical application. Drug Deliv. 2022, 29, 2296–2319. [Google Scholar] [CrossRef]












| Influence Factors | Performance | Mechanism |
|---|---|---|
| LA: GA ratio | The higher the LA ratio, the slower the degradation | The higher the percentage of LA, the more hydrophobic it is and the slower the degradation rate |
| End group | Acid-terminated degrades more quickly than ester-terminated | Highly hydrophobic PLGA with ester-capped end |
| Molecular weight | The higher the molecular weight, the slower the degradation | The larger the molecular weight, the longer the polymer chain and the slower the degradation |
| pH | Slower rate of degradation under alkaline conditions compared to acidic conditions | The H+ produced by degradation is neutralized with OH− in the environment, making the autocatalytic effect of -COOH weaker |
| Temperature | The higher the temperature, the faster the degradation rate | Increased temperature promotes hydration layer formation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. https://doi.org/10.3390/ph16030454
Lu Y, Cheng D, Niu B, Wang X, Wu X, Wang A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals. 2023; 16(3):454. https://doi.org/10.3390/ph16030454
Chicago/Turabian StyleLu, Yue, Dongfang Cheng, Baohua Niu, Xiuzhi Wang, Xiaxia Wu, and Aiping Wang. 2023. "Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research" Pharmaceuticals 16, no. 3: 454. https://doi.org/10.3390/ph16030454