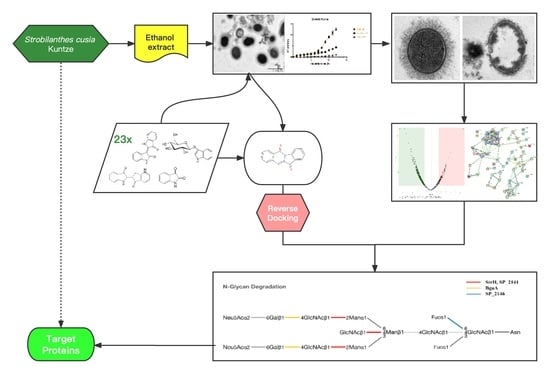
Combining the In Silico and In Vitro Assays to Identify Strobilanthes cusia Kuntze Bioactives against Penicillin-Resistant Streptococcus pneumoniae
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity of ES In Vitro
2.1.1. Antibacterial Effect against Standard Strains
2.1.2. Antibacterial Activity against Multidrug-Resistant Clinical Isolates
2.2. ES Inhibits the Growth of PRSP F3983
2.2.1. The Growth Curves of F3983 Treated by ES
2.2.2. ES Effects on Membrane Integrity, Cell Wall, and Capsule Formation of F3983
2.3. Proteomics Analysis of PRSP F3983 Treated by ES
2.3.1. Differentially Expressed Proteins (DEPs)
2.3.2. Protein–Protein Interactions (PPI) Analysis for Down-Regulated DEPs
2.3.3. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis of the Down-Regulated DEPs
2.4. Chemical Contents of S. cusia Leaves and Antibacterial Effects on the PRSP F3983
2.4.1. Chemical Contents of S. cusia Leaves
2.4.2. MIC Tests for the Isolated 23 Chemical Compounds on the PRSP F3983
2.5. Anti-PRSP Effects of Tryptanthrin and Its Potential Targets
2.5.1. Antibacterial Activity of Tryptanthrin
2.5.2. Target Screening In Silico: Reverse Docking for Tryptanthrin with Known Structure Proteins of S. pneumoniae
2.6. Visualization of Tryptanthrin with Its Potential Targets by Discovery Studio
2.7. Interaction of Tryptanthrin with Three Potential Targets on the N-Glycan Pathway of PRSP by Surface Plasmon Resonance (SPR) Assay
3. Discussion
4. Materials and Methods
4.1. Preparation of Ethanol Extract of S. cusia Leaves
4.1.1. Samples Collection
4.1.2. Sample Preparation
4.2. UPLC-UV-ESI-Q-TOF/MS Analysis of S. cusia Leaves
4.3. Preparation of Isolated Compounds from S. cusia Leaves
4.4. Bacterial Strains and Growth Conditions
4.5. Minimal Inhibitory Concentration (MIC) Assay
4.6. Whole Genome Sequencing of PRSP (Strain F3983)
4.6.1. Library Preparation and Sequencing
4.6.2. Bioinformatic Procedures
4.7. Growth Curves of the PRSP F3983
4.8. Transmission Electron Microscopy (TEM)
4.9. Bacterial Viability Assay (Confocal)
4.10. Proteomics Analysis
4.10.1. Sample Preparation
4.10.2. Nano-LC–MS/MS Analysis by Q-Exactive plus Orbitrap
4.10.3. Protein Identification and Data Analysis
4.11. Targets Screening for Tryptanthrin by High-Throughput Reverse Docking
4.11.1. Preparation of Tryptanthrin and Protein Structure
4.11.2. Reverse Docking Using AutoDock Vina
4.12. Protein Expression and Purification
4.12.1. Cloning
4.12.2. Protein Extraction
4.13. Surface Plasmon Resonance (SPR) Assay to Evaluate the Protein Binding Affinity
4.14. Molecular Docking and Visualization
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cámara, M.; Boulnois, G.J.; Andrew, P.W.; Mitchell, T.J. A neuraminidase from Streptococcus pneumoniae has the features of a surface protein. Infect. Immun. 1994, 62, 3688–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, E.; Camilli, A. ManLMN is a glucose transporter and central metabolic regulator in Streptococcus pneumoniae. Mol. Microbiol. 2016, 102, 467–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.M.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.M.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.A.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef] [Green Version]
- Sender, V.; Hentrich, K.; Henriques-Normark, B. Virus-Induced Changes of the Respiratory Tract Environment Promote Secondary Infections with Streptococcus pneumoniae. Front. Cell Infect. Microbiol. 2021, 11, 643326. [Google Scholar] [CrossRef]
- Ko, H.C.; Wei, B.L.; Chiou, W.F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006, 107, 205–210. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.; Ran, Q.; Huang, Q.W.; Wang, J. Strobilanthes cusia (Nees) Kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: A comprehensive review. J. Ethnopharmacol. 2021, 265, 113325. [Google Scholar] [CrossRef]
- Sun, Q.; Leng, J.; Tang, L.; Wang, L.; Fu, C. A Comprehensive Review of the Chemistry, Pharmacokinetics, Pharmacology, Clinical Applications, Adverse Events, and Quality Control of Indigo Naturalis. Front. Pharmacol. 2021, 12, 664022. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, C.-L.; Yen, H.-R.; Chang, Y.-S.; Lin, Y.-P.; Huang, S.-H.; Lin, C.-W. Antiviral Action of Tryptanthrin Isolated from Strobilanthes cusia Leaf against Human Coronavirus NL63. Biomolecules 2020, 10, 336. [Google Scholar] [CrossRef]
- Chiang, Y.R.; Li, A.; Leu, Y.L.; Fang, J.Y.; Lin, Y.K. An In Vitro Study of the Antimicrobial Effects of Indigo Naturalis Prepared from Strobilanthes formosanus Moore. Molecules 2013, 18, 14381–14396. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.Y.; Chen, X.W.; Zhou, W.P.; Liao, L. Effects of Baphicacanthus cusia (Nees) Bremek extract on the antibacterial activity of lincomycin in vitro. J. Anhui Agric. Sci. 2010, 38, 2927–2928. [Google Scholar]
- Honda, G.; Tabata, M.; Tsuda, M. The antimicrobial specificity of tryptanthrin. Planta Med. 1979, 37, 172–174. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, Y.; Hao, X.-J.; Yang, F.-M.; Sun, Q.-Y.; Morris-Natschke, S.L.; Lee, K.-H.; Wang, Y.-H.; Long, C.-L. Indole alkaloid glycosides from the aerial parts of Strobilanthes cusia. J. Nat. Prod. 2014, 77, 2590–2594. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Luo, X.; Hu, L.; Lu, H. Functional metabolomics innovates therapeutic discovery of traditional Chinese medicine derived functional compounds. Pharmacol. Ther. 2021, 224, 107824. [Google Scholar] [CrossRef]
- Gao, T.; Ye, F.; Tan, Y.; Peng, M.; Yuan, F.; Liu, Z.; Zhou, D.; Yang, K.; Liu, W.; Guo, R.; et al. Metabolomics and proteomics analyses revealed mechanistic insights on the antimicrobial activity of epigallocatechin gallate against Streptococcus suis. Front. Cell Infect. Microbiol. 2022, 12, 973282. [Google Scholar] [CrossRef]
- Deng, H.; Kong, Y.; Zhu, J.; Jiao, X.; Tong, Y.; Wan, M.; Zhao, Y.; Lin, S.; Ma, Y.; Meng, X. Proteomic analyses revealed the antibacterial mechanism of Aronia melanocarpa isolated anthocyanins against Escherichia coli O157: H7. Curr. Res. Food Sci. 2022, 5, 1559–1569. [Google Scholar] [CrossRef]
- Lv, N.; Kong, Q.; Zhang, H.; Li, J. Discovery of novel Staphylococcus aureus penicillin binding protein 2a inhibitors by multistep virtual screening and biological evaluation. Bioorg. Med. Chem. Lett. 2021, 41, 128001. [Google Scholar] [CrossRef]
- Galati, S.; Di Stefano, M.; Martinelli, E.; Poli, G.; Tuccinardi, T. Recent Advances in In Silico Target Fishing. Molecules 2021, 26, 5124. [Google Scholar] [CrossRef]
- Kuhnert, N.; Yassin, G.H.; Jaiswal, R.; Matei, M.F.; Grün, C.H. Differentiation of prototropic ions in regioisomeric caffeoyl quinic acids by electrospray ion mobility mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 675–680. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, L.; Gong, K.; Zhi, X.; Guan, Y.; Cai, W. Identification of the Constituents of Percutaneous Absorption from Duhaldea nervosa Based on UHPLC-Q-Exactive Orbitrap MS and Microdialysis Technique. Int. J. Anal. Chem. 2019, 2019, 8328942. [Google Scholar] [CrossRef] [PubMed]
- Garayev, E.; Di Giorgio, C.; Herbette, G.; Mabrouki, F.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. Bioassay-guided isolation and UHPLC-DAD-ESI-MS/MS quantification of potential anti-inflammatory phenolic compounds from flowers of Inula montana L. J. Ethnopharmacol. 2018, 226, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Molino, R.; Junio, H.A. Profiling the Philippine Blue: Liquid chromatography/mass spectrometry-based metabolomics study on Philippine Indigofera. Rapid Commun. Mass Spectrom. 2021, 35, e9037. [Google Scholar] [CrossRef] [PubMed]
- Oberthur, C.; Jaggi, R.; Hamburger, M. HPLC based activity profiling for 5-lipoxygenase inhibitory activity in Isatis tinctoria leaf extracts. Fitoterapia 2005, 76, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chang, H.; Wang, L.; Wu, S.; Shao, B.; Zhou, J.; Zhao, Y. Detection, Occurrence and Fate of Indirubin in Municipal Sewage Treatment Plants. Environ. Sci. Technol. 2008, 42, 8339–8344. [Google Scholar] [CrossRef]
- Liau, B.C.; Jong, T.-T.; Lee, M.-R.; Chen, S.-S. LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in daqingye and banlangen. J. Pharm. Biomed. Anal. 2007, 43, 346–351. [Google Scholar] [CrossRef]
- Costa, D.C.M.; de Azevedo, M.M.B.; e Silva, D.O.; Romanos, M.T.V.; Souto-Padrón, T.C.B.S.; Alviano, C.S.; Alviano, D.S. In vitro anti-MRSA activity of Couroupita guianensis extract and its component Tryptanthrin. Nat. Prod. Res. 2017, 31, 2077–2080. [Google Scholar] [CrossRef]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef]
- Ruiz-Moreno, A.J.; Domling, A.; Velasco-Velazquez, M.A. Reverse Docking for the Identification of Molecular Targets of Anticancer Compounds. Methods Mol. Biol. 2021, 2174, 31–43. [Google Scholar]
- Vollmer, W.; Massidda, O.; Tomasz, A. The Cell Wall of Streptococcus pneumoniae. Microbiol. Spectr. 2019, 7, GPP3-0018-2018. [Google Scholar] [CrossRef]
- Paixao, L.; Oliveira, J.; Veríssimo, A.; Vinga, S.; Ventura, M.R.; Kjos, M.; Veening, J.-W.; Fernandes, V.E.; Andrew, P.W.; Yesilkaya, H.; et al. Host glycan sugar-specific pathways in Streptococcus pneumoniae: Galactose as a key sugar in colonisation and infection [corrected]. PLoS ONE 2015, 10, e0121042. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, J.K.; Pluvinage, B.; Boraston, A.B. Glycan-metabolizing enzymes in microbe-host interactions: The Streptococcus pneumoniae paradigm. FEBS Lett. 2018, 592, 3865–3897. [Google Scholar] [CrossRef]
- Paixao, L.; Caldas, J.; Kloosterman, T.G.; Kuipers, O.P.; Vinga, S.; Neves, A.R. Transcriptional and metabolic effects of glucose on Streptococcus pneumoniae sugar metabolism. Front. Microbiol. 2015, 6, 1041. [Google Scholar] [CrossRef]
- Hartel, T.; Eylert, E.; Schulz, C.; Petruschka, L.; Gierok, P.; Grubmüller, S.; Lalk, M.; Eisenreich, W.; Hammerschmidt, S. Characterization of central carbon metabolism of Streptococcus pneumoniae by isotopologue profiling. J. Biol. Chem. 2012, 287, 4260–4274. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.M.; Kuipers, O.P.; Neves, A.R. Environmental and nutritional factors that affect growth and metabolism of the pneumococcal serotype 2 strain D39 and its nonencapsulated derivative strain R6. PLoS ONE 2013, 8, e58492. [Google Scholar] [CrossRef] [Green Version]
- Troxler, L.J.; Werren, J.P.; Schaffner, T.O.; Mostacci, N.; Vermathen, P.; Vermathen, M.; Wüthrich, D.; Simillion, C.; Brugger, S.D.; Bruggmann, R.; et al. Carbon source regulates polysaccharide capsule biosynthesis in Streptococcus pneumoniae. J. Biol. Chem. 2019, 294, 17224–17238. [Google Scholar] [CrossRef] [Green Version]
- Panjaitan, N.S.D.; Hong, Y.-T.; Chien, C.-C.; Yang, H.-C.; You, R.-I.; Soo, P.-C. The PTS Components in Klebsiella pneumoniae Affect Bacterial Capsular Polysaccharide Production and Macrophage Phagocytosis Resistance. Microorganisms. 2021, 9, 335. [Google Scholar] [CrossRef]
- Afzal, M.; Shafeeq, S.; Kuipers, O.P. LacR is a repressor of lacABCD and LacT is an activator of lacTFEG, constituting the lac gene cluster in Streptococcus pneumoniae. Appl. Environ. Microbiol. 2014, 80, 5349–5358. [Google Scholar] [CrossRef] [Green Version]
- Bidossi, A.; Mulas, L.; Decorosi, F.; Colomba, L.; Ricci, S.; Pozzi, G.; Deutscher, J.; Viti, C.; Oggioni, M.R. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE 2012, 7, e33320. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.M.; Kloosterman, T.G.; Kuipers, O.P.; Neves, A.R. CcpA ensures optimal metabolic fitness of Streptococcus pneumoniae. PLoS ONE 2011, 6, e26707. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. 5-O-Caffeoylquinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT Food Sci. Technol. 2016, 65, 471–479. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Lee, K.; Kim, D. Using reverse docking for target identification and its applications for drug discovery. Expert. Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Burnaugh, A.M.; Frantz, L.J.; King, S.J. Growth of Streptococcus pneumoniae on human glycoconjugates is dependent upon the sequential activity of bacterial exoglycosidases. J. Bacteriol. 2008, 190, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Gregg, K.J.; Zandberg, W.F.; Hehemann, J.-H.; Whitworth, G.E.; Deng, L.; Vocadlo, D.J.; Boraston, A.B. Analysis of a new family of widely distributed metal-independent alpha-mannosidases provides unique insight into the processing of N-linked glycans. J. Biol. Chem. 2011, 286, 15586–15596. [Google Scholar] [CrossRef] [Green Version]
- Dalia, A.B.; Standish, A.J.; Weiser, J.N. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect. Immun. 2010, 78, 2108–2116. [Google Scholar] [CrossRef] [Green Version]
- King, S.J. Pneumococcal modification of host sugars a major contributor to colonization of the airway? Mol. Oral Microbiol. 2010, 25, 15–24. [Google Scholar] [CrossRef]
- King, S.J.; Hippe, K.R.; Weiser, J.N. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 2006, 59, 961–974. [Google Scholar] [CrossRef]
- Singh, A.K.; Pluvinage, B.; Higgins, A.M.; Dalia, B.A.; Woodiga, A.S. Unravelling the multiple functions of the architecturally intricate Streptococcus pneumoniae beta-galactosidase, BgaA. PLoS Pathog. 2014, 10, e1004364. [Google Scholar] [CrossRef]
- Harty, D.W.; Chen, Y.; Simpson, C.L.; Berg, T.; Cook, S.L.; Mayo, J.A.; Hunter, N.; Jacques, N.A. Characterisation of a novel homodimeric N-acetyl-beta-D-glucosaminidase from Streptococcus gordonii. Biochem. Biophys. Res. Commun. 2004, 319, 439–447. [Google Scholar] [CrossRef]
- Robb, M.; Robb, C.S.; Higgins, M.A.; Hobbs, J.K.; Paton, J.C.; Boraston, A.B. A Second beta-Hexosaminidase Encoded in the Streptococcus pneumoniae Genome Provides an Expanded Biochemical Ability to Degrade Host Glycans. J. Biol. Chem. 2015, 290, 30888–30900. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, J.K.; Pluvinage, B.; Robb, M.; Smith, S.P.; Boraston, A.B. Two complementary alpha-fucosidases from Streptococcus pneumoniae promote complete degradation of host-derived carbohydrate antigens. J. Biol. Chem. 2019, 294, 12670–12682. [Google Scholar] [CrossRef]
- Robb, M.; Hobbs, J.K.; Woodiga, S.A.; Shapiro-Ward, S.; Suits, M.D.; McGregor, N.; Brumer, H.; Yesilkaya, H.; King, S.J.; Boraston, A.B. Molecular Characterization of N-glycan Degradation and Transport in Streptococcus pneumoniae and Its Contribution to Virulence. PLoS Pathog. 2017, 13, e1006090. [Google Scholar] [CrossRef] [Green Version]
- Janesch, P.; Rouha, H.; Badarau, A.; Stulik, L.; Mirkina, I.; Caccamo, M.; Havlicek, K.; Maierhofer, B.; Weber, S.; Groß, K.; et al. Assessing the function of pneumococcal neuraminidases NanA, NanB and NanC in in vitro and in vivo lung infection models using monoclonal antibodies. Virulence 2018, 9, 1521–1538. [Google Scholar] [CrossRef] [Green Version]
- Leber, A.L. Broth microdilution test. In Clinical Microbiology Procedures Handbook; American Society for Microbiology: Washington, DC, USA; Wiley: New York, NY, USA, 2016; pp. 5.2.1.1–5.2.2.10. [Google Scholar]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.; Birol, I. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009, 19, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Han, D. BWA-MEME: BWA-MEM emulated with a machine learning approach. Bioinformatics 2022, 38, 2404–2413. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, S.; Rohde, M. Electron Microscopy to Study the Fine Structure of the Pneumococcal Cell. Methods Mol. Biol. 2019, 1968, 13–33. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Lin, J.; Von Mering, C.; Jensen, L.J. SVD-phy: Improved prediction of protein functional associations through singular value decomposition of phylogenetic profiles. Bioinformatics 2016, 32, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33, D433–D437. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Jao, C.; Lin, W.; Wu, Y.; Wu, P. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef]
- Barnes, J.P.; Putnam, A.R.; Burke, B.A.; Aasen, A.J. Isolation and characterization of allelochemicals in rye herbage. Phytochemistry 1987, 26, 1385–1390. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Dellagreca, M.; Fiorentino, A.; Monaco, P.; Oriano, P.; Temussi, F. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar] [CrossRef]
- Abdolali, A.; Jvad, M. Unexpected approach to the synthesis of 2-phenyl quinoxalines and pyrido[2,3-b]pyrazines via a regioselective reaction. Helv. Chim. Acta 2013, 96, 124–129. [Google Scholar]
- Koálová, D.; Hrochová, V.; Suchý, V.; Buděšínský, M.; Ubik, K. Two pyrrole acids from Berberis koreana. Phytochemistry 1992, 31, 3669–3670. [Google Scholar] [CrossRef]
- Katade, S.R.; Pawar, P.V.; Tungikar, V.B.; Tambe, A.; Kalal, K.; Wakharkar, R.; Deshpande, N. Larvicidal activity of bis (2-ethylhexyl) benzene-1,2-dicarboxylate from Sterculia guttata seeds against two mosquito species. Chem. Biodivers. 2006, 3, 49–53. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, H.; Li, G.; Wu, H. A new triterpenoid from Entodon okamurae broth. J. Asian Nat. Prod. Res. 2003, 5, 189–195. [Google Scholar] [CrossRef]
- Wu, P.; Hsu, Y.; Jao, C. Indole alkaloids from Cephalanceropsis gracilis. J. Nat. Prod. 2006, 69, 1467–1470. [Google Scholar] [CrossRef]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and phytotoxicity of apocarotenoids from Chenopodium album. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Gutierrez-Lugo, M.; Woldemichael, G.M.; Singh, M.P.; Suarez, P.; Maiese, W.; Montenegro, G.; Timmermann, B. Isolation of three new naturally occurring compounds from the culture of Micromonospora sp. P1068. Nat. Prod. Res. 2005, 19, 645–652. [Google Scholar] [CrossRef]
- Duan, H.; Takaishi, Y.; Momota, H.; Ohmoto, Y.; Taki, T. Immunosuppressive constituents from Saussurea medusa. Phytochemistry 2002, 59, 85–90. [Google Scholar] [CrossRef]
- Jones, L.; Bartholomew, B.; Latif, Z.; Sarker, S.; Nash, R. Constituents of Cassia laevigata. Fitoterapia 2000, 71, 580–583. [Google Scholar] [CrossRef]
- Liu, Y.H.; Qing, G.W.; Ding, S.P.; Wu, X.Y. Studies on chemical constituents in root of Isatis indigotica. Chin. Tradit. Herb. Drugs 2002, 33, 97–99. [Google Scholar]
- Yang, Q.; Ye, G. A new c-glucoside from Commelina communis. Chem. Nat. Compd. 2009, 45, 59–60. [Google Scholar] [CrossRef]
- Marques, M.R.; Stüker, C.; Kichik, N.; Tarragó, T.; Giralt, E.; Morel, A.; Dalcol, I. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia 2010, 81, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.M.; David, M.; Alberto, O.; Chinchilla, D.; Simonet, A.M.; Molinillo, J.M.G. Isolation and synthesis of allelochemicals from gramineae: Benzoxazinones and related compounds. J. Agric. Food Chem. 2006, 54, 991–1000. [Google Scholar]
- Xie, H.; Liang, Y.; Xue, J. Secondary metabolites of the phytopathogen Peronophythora litchii. Nat. Prod. Commun. 2010, 5, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Hou, A.; Mei, S.; Mei, S.-X.; Sun, H.-D.; Che, C.-T. Constituents of Clerodendrum bungei. J. Asian Nat. Prod. Res. 2002, 4, 165–169. [Google Scholar] [CrossRef]
- Hacıbekiroğlu, I.; Kolak, U. Antioxidant and anticholinesterase constituents from the petroleum ether and chloroform extracts of Iris suaveolens. Phytother. Res. 2011, 25, 522–529. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, C.L.; Wang, Y.; Zhang, X.Q.; Huang, X.J.; Ye, W.C. Chemical constituents from roots of Isatis indigotica. Zhongguo Zhong Yao Za Zhi 2018, 43, 2091–2096. [Google Scholar]
- Yang, S.Y.; Li, Z.L.; Zhao, Y.M.; Zuo, Q.Y.; Lv, X.H.; Wang, X.J.; Qian, S.H. Chemical constituents from flowers of Abelmoschus manihot. Chin. Tradit. Herb. Drugs. 2017, 48, 2827–2842. [Google Scholar]
- Liu, Y.; Ou, Y.F.; Yao, X.S. Chemical constituents in the leaves of Baphicacanthus cusia (Nees) Bremek. Chin. J. Med. Chem. 2009, 19, 273–275. [Google Scholar]
- Xie, P.; Zhang, Y.; Wang, X.; Wei, J.; Kang, W. Antithrombotic effect and mechanism of Rubus spp. Blackberry. Food Funct. 2017, 8, 2000–2012. [Google Scholar] [CrossRef]








| MICs (µg/mL) of ES | ||||||
|---|---|---|---|---|---|---|
| Bacteria /Strain | Gram-positive bacteria | Gram-negative bacteria | ||||
| Staphylococcus aureus ATCC29213 | Streptococcus pneumoniae ATCC49619 | Pseudomonas aeruginosa ATCC27853 | Klebsiella pneumoniae ATCC13883 | Acinetobacter baumannii ATCC19606 | Enterococcus faecalis ATCC29212 | |
| MIC | 100 | 200 | >800 | >800 | >800 | >800 |
| MICs (µg/mL) of ES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Penicillin-resistant Streptococcus pneumonia (PRSP) | Methicillin-resistant Staphylococcus aureus (MRSA) | ||||||||
| Strain | F3368 | F3401 | F3755 | F3983 | 48900 | 49008 | 48973 | 49025 | 48966 | 48706 |
| MIC | 200 | 200 | 200 | 200 | >800 | >800 | >800 | >800 | >800 | >800 |
| MICs (µg/mL) of Seven CRSs | ||||||
|---|---|---|---|---|---|---|
| 3-CQA | 4-CQA | 5-CQA | Hispiduloside | Tryptanthrin | Hispidulin | Indirubin |
| 200 | 200 | 200 | 200 | 25 | 100 | >800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Jin, L.; Zhao, Z.; Xu, X.; Liu, S.; Huang, Y.; Liu, X.; Xu, Y.; Yang, D.; Huang, W.; et al. Combining the In Silico and In Vitro Assays to Identify Strobilanthes cusia Kuntze Bioactives against Penicillin-Resistant Streptococcus pneumoniae. Pharmaceuticals 2023, 16, 105. https://doi.org/10.3390/ph16010105
Han X, Jin L, Zhao Z, Xu X, Liu S, Huang Y, Liu X, Xu Y, Yang D, Huang W, et al. Combining the In Silico and In Vitro Assays to Identify Strobilanthes cusia Kuntze Bioactives against Penicillin-Resistant Streptococcus pneumoniae. Pharmaceuticals. 2023; 16(1):105. https://doi.org/10.3390/ph16010105
Chicago/Turabian StyleHan, Xiaoyu, Lu Jin, Zhimin Zhao, Xinjun Xu, Shiyi Liu, Yuquan Huang, Xiaoli Liu, Yuehong Xu, Depo Yang, Wei Huang, and et al. 2023. "Combining the In Silico and In Vitro Assays to Identify Strobilanthes cusia Kuntze Bioactives against Penicillin-Resistant Streptococcus pneumoniae" Pharmaceuticals 16, no. 1: 105. https://doi.org/10.3390/ph16010105