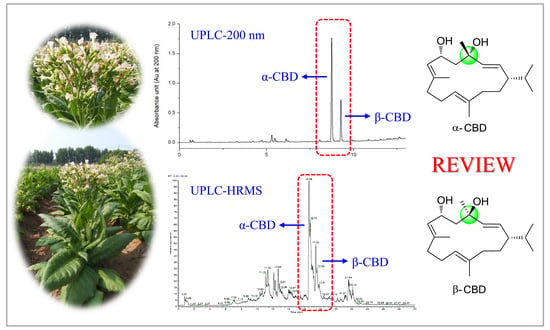
Structural Modifications and Biological Activities of Natural α- and β-Cembrenediol: A Comprehensive Review
Abstract
:1. Introduction
2. Method of Searching Literature
3. Structural Modifications of α- and β-CBD
3.1. Chemical Synthesis
3.2. Biotransformation
4. Biological Properties of α-/β-CBD and Its Analogs
4.1. Antitumor Activity
4.2. Neuroprotective Activity
4.3. Antimicrobial Activity
5. Patent Release of α-/β-CBD and Their Analogs
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, S.; Lim, U.T. Extract of Nicotiana tabacum as a potential control agent of Grapholita molesta (Lepidoptera: Tortricidae). PLoS ONE 2018, 13, e0198302. [Google Scholar] [CrossRef] [Green Version]
- Millan, D.C.; Shiogiri, N.S.; da Silva de Souza, N.E.; Da Silva, H.R.; Fernandes, M.N. Ecotoxicity and hematological effects of a natural insecticide based on tobacco (Nicotiana tabacum) extract on Nile tilapia (Oreochromis niloticus). Acta Sci. Biol. Sci. 2013, 35, 157–162. [Google Scholar] [CrossRef]
- Akumefula, M.I.; Onwusonye, J.; Osuji, C.N.; Uzomba, O.D.A.; Akumefula, F.U.; Ubaka, K.; Eziukwu, C. Comparative assessment of the insecticidal potency of tobacco leaves extract (Nicotiana tabacum), black pepper seeds (Uziza) extract (Piper guineense) and African pepper seeds (Uda) extract (Xylapia aetiopica). Chem. Mater. Res. 2014, 6, 57–59. [Google Scholar]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological roles and biological activities of specialized metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Roberts, D.L.; Rowland, R.L. Macrocyclic diterpenes. α- and β-4,8,13-duvatriene-1,3-diols from tobacco. J. Org. Chem. 1962, 27, 3989–3995. [Google Scholar]
- Springer, J.P.; Clardy, J.; Cox, R.H.; Cutler, H.G.; Cole, R.J. The structure of a new type of plant growth inhibitor extracted from immature tobacco leaves. Tetrahedron Lett. 1975, 16, 2737–2740. [Google Scholar] [CrossRef]
- Begley, M.J.; Crombie, L.; McNamara, D.; Firth, D.F.; Smith, S.; Bevan, P.C. Cembrenediols in the curing of tobacco. X-ray crystal structures of β-cembrenediol and α-cembreneketol. Phytochemistry 1988, 27, 1695–1703. [Google Scholar] [CrossRef]
- Marshall, J.A.; Robinson, E.D.; Adams, R.D. Stereoselective total synthesis of β-2,7,11-Cembratriene-4, 6-diol (β-CBT), a tumor inhibitory constituent of tobacco smoke. Tetrahedron Lett. 1988, 29, 4913–4916. [Google Scholar] [CrossRef]
- Marshall, J.A.; Robinson, E.D. Enantioselective total synthesis of (+)-α-2,7,11-cembratriene-4,6-diol (α-CBT). Tetrahedron Lett. 1989, 30, 1055–1058. [Google Scholar] [CrossRef]
- Marshall, J.A.; Robinson, E.D.; Lebreton, J. Synthesis of the tumor inhibitory tobacco constituents α- and β-2,7,11-cembratriene-4,6-diol by diastereoselective [2,3] Wittig ring contraction. J. Org. Chem. 1990, 55, 227–239. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Li, X.L.; Chen, Z.Y.; Liu, Q.B.; Zhu, X.L.; Yang, J. Determination of cembrenediols in tobacco by gas chromatography-mass spectrometry-selected ion monitoring with precolumn derivatization. Acta Chromatogr. 2016, 28, 513–524. [Google Scholar] [CrossRef] [Green Version]
- El Sayed, K.A.; Sylvester, P.W. Biocatalytic and semisynthetic studies of the anticancer tobacco cembranoids. Expert Opin. Investig. Drugs 2007, 16, 877–887. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, P.; Gong, D.; Zhang, Z. Chemical structures, biosynthesis, bioactivities, biocatalysis and semisynthesis of tobacco cembranoids: An overview. Ind. Crops Prod. 2016, 83, 66–80. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, Z. A review on bioactivities of tobacco cembranoid diterpenes. Biomolecules 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.F.; Hua, T.; Huang, S.; Yang, J.; Wei, T.; Mao, D.B. Progress on cembranoid diterpenes in Nicotiana tabacum L. Chem. Reag. 2020, 53, 1689–1699. [Google Scholar]
- Soosaraei, M.; Khasseh, A.A.; Fakhar, M.; Hezarjaribi, H.Z. A decade bibliometric analysis of global research on leishmaniasis in Web of Science database. Ann. Med. Surg. 2018, 26, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Lin, M.H.; Hwang, I.H.; Chen, T.J.; Lin, H.C.; Hou, M.C.; Hwang, S.J. Scientific publications in gastroenterology and hepatology in Taiwan: An analysis of Web of Science from 1993 to 2013. J. Chin. Med. Assoc. 2017, 80, 80–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Takizawa, H.; Konishi, S.; Yoshida, D.; Mizusaki, S. Identification of cembratriene-4, 6-diol as antitumor-promoting agent from cigarette smoke condensate. Carcinogenesis 1985, 6, 1189–1194. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Laphookhieo, S.; Yousaf, M.; Prestridge, J.A.; Shirode, A.B.; Wali, V.B.; Sylvester, P.W. Semisynthetic and biotransformation studies of (1S,2E,4S,6R,7E,11E)-2,7,11-cembratriene-4,6-diol. J. Nat. Prod. 2008, 71, 117–122. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Laphookhieo, S.; Baraka, H.N.; Yousaf, M.; Hebert, A.; Bagaley, D.; Rainey, F.A.; Muralidharan, A.; Thomas, S.; Shah, G.V. Biocatalytic and semisynthetic optimization of the anti-invasive tobacco (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol. Bioorg. Med. Chem. 2008, 16, 2886–2893. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Mohyeldin, M.M.; Hailat, M.M.; El Sayed, K.A. (1S, 2E,4S,7E,11E)-2,7,11-Cembratriene-4,6-diol semisynthetic analogs as novel c-Met inhibitors for the control of c-Met-dependent breast malignancies. Bioorg. Med. Chem. 2016, 24, 5748–5761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, B.; Wang, L.; Xu, G.; Yang, P.; Zhang, G.; Mao, D. Synthesis and antitumor activities of α-2,7,11-cembratriene-4,6-diol derivatives. Chem. J. Chin. Univ. 2020, 41, 481–489. [Google Scholar]
- Jiang, H.; Qin, X.; Wang, Q.; Xu, Q.; Wang, J.; Wu, Y.; Chen, W.; Wang, C.; Zhang, T.; Xing, D.; et al. Application of carbohydrates in approved small molecule drugs: A review. Eur. J. Med. Chem. 2021, 223, 113633. [Google Scholar] [CrossRef]
- Paulsen, H.; Lê-Nguyên, B.; Sinnwell, V.; Heemann, V.; Seehofer, F. Synthesis of glycosides of mono-, sesqui-, and diterpene alcohols. Liebigs Ann. Chem. 1985, 8, 1513–1536. [Google Scholar] [CrossRef]
- Baraka, H.N.; Khanfar, M.A.; Williams, J.C.; El-Giar, E.M.; El Sayed, K.A. Bioactive natural, biocatalytic, and semisynthetic tobacco cembranoids. Planta Med. 2011, 77, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Arnarp, J.; Chu, W.L.A.; Enzell, C.R.; Hewitt, G.M.; Kutney, J.P.; Li, K.; Milanova, R.K.; Nakata, H.; Nasiri, A.; Tsuda, T. Tobacco chemistry. Part 77. Biotransformations of a major tobacco cembratrienediol using plant cell cultures of Nicotiana sylvestris. Acta Chem. Scand. 1993, 47, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Arnarp, J.; Chu, W.L.A.; Enzell, C.R.; Hewitt, G.M.; Kutney, J.P.; Li, K.; MIlanova, R.K.; Nakata, H.; Nasiri, A.; Okada, Y. Tobacco chemistry. 76. Biotransformations of tobacco isoprenoids using plant cell cultures of Tripterygium wilfordii. Acta Chem. Scand. 1993, 47, 683–688. [Google Scholar] [CrossRef]
- Hailat, M.M.; Ebrahim, H.Y.; Mohyeldin, M.M.; Goda, A.A.; Siddique, A.B.; El Sayed, K.A. The tobacco cembranoid (1S,2E,4S,7E,11E)-2,7,11-cembratriene-4,6-diol as a novel angiogenesis inhibitory lead for the control of breast malignancies. Bioorg. Med. Chem. 2017, 25, 3911–3921. [Google Scholar] [CrossRef]
- Yuan, X.L.; Mao, X.X.; Du, Y.M.; Yan, P.Z.; Hou, X.D.; Zhang, Z.F. Anti-tumor activity of cembranoid-type diterpenes isolated from Nicotiana tabacum L. Biomolecules 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Culver, P.; Jacobs, R.S. Lophotoxin: A neuromuscular acting toxin from the sea whip (Lophogorgia rigida). Toxicon 1981, 19, 825–830. [Google Scholar] [CrossRef]
- Fenical, W.; Okuda, R.K.; Bandurraga, M.M.; Culver, P.; Jacobs, R.S. Lophotoxin: A novel neuromuscular toxin from Pacific sea whips of the genus Lophogorgia. Science 1981, 212, 1512–1514. [Google Scholar] [CrossRef] [PubMed]
- Ferchmin, P.A.; Lukas, R.J.; Hann, R.M.; Fryer, J.D.; Eaton, J.B.; Pagan, O.R.; Rodriguez, A.D.; Nicolau, Y.; Rosado, M.; Cortes, S.; et al. Tobacco cembranoids block behavioral sensitization to nicotine and inhibit neuronal acetylcholine receptor function. J. Neurosci. Res. 2001, 64, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.J.; Ospina, C.A.; Rodriguez, A.D.; Eterovic, V.A. Differential inhibition of nicotine- and acetylcholineevoked currents through alpha4beta2 neuronal nicotinic receptors by tobacco cembranoids in Xenopus oocytes. Neurosci. Lett. 2004, 366, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ferchmin, P.A.; Pagán, O.R.; Ulrich, H.; Szeto, A.C.; Hann, R.M.; Eterović, V.A. Actions of octocoral and tobacco cembranoids on nicotinic receptors. Toxicon 2009, 54, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Ferchmin, P.A.; Hao, J.; Perez, D.; Penzo, M.; Maldonado, H.M.; Gonzalez, M.T.; Rodriguez, A.D.; de Vellis, J. Tobacco cembranoids protect the function of acute hippocampal slices against NMDA by a mechanism mediated by α4β2 nicotinic receptors. J. Neurosci. Res. 2005, 82, 631–641. [Google Scholar] [CrossRef]
- Eterovic, V.A.; Perez, D.; Martins, A.H.; Cuadrado, B.L.; Carrasco, M.; Ferchmin, P.A. A cembranoid protects acute hippocampal slices against paraoxon neurotoxicity. Toxicol. Vitr. 2011, 25, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Ferchmin, P.; Andino, M.; Salaman, R.R.; Alves, J.; Velez-Roman, J.; Cuadrado, B.; Carrasco, M.; Torres-Rivera, W.; Segarra, A.; Martins, A.H.; et al. 4R-cembranoid protects against diisopropylfluorophosphate-mediated neurodegeneration. Neurotoxicology 2014, 44, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Eterovic, V.A.; Del Valle-Rodriguez, A.; Perez, D.; Carrasco, M.; Khanfar, M.A.; El Sayed, K.A.; Ferchmin, P.A. Protective activity of (1S,2E,4R,6R,7E,11E)-2,7,11-cembratriene-4,6-diol analogues against diisopropylfluorophosphate neurotoxicity: Preliminary structure-activity relationship and pharmacophore modeling. Bioorg. Med. Chem. 2013, 21, 4678–4686. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Ferchmin, P.A.; Hemmerle, A.M.; Seroogy, K.B.; Eterovic, V.A.; Hao, J. 4R-Cembranoid improves outcomes after 6-hydroxydopamine challenge in both in vitro and in vivo models of Parkinson’s disease. Front. Neurosci. 2017, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Candelario-Jalil, E.; Mhadu, N.; Gonzalez-Falcon, A.; Garcia-Cabrera, M.; Munoz, E.; Leon, O.; Fiebich, B. Effects of the cyclooxygenase-2 inhibitor nimesulide on cerebral infarction and neurological deficits induced by permanent middle cerebral artery occlusion in the rat. J. Neuroinflam. 2005, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Olsson, E.; Holth, A.; Kumlin, E.; Bohlin, L.; Wahlberg, I. Structure-related inhibiting activity of some tobacco cembranoids on the prostaglandin synthesis in vitro. Planta Med. 1993, 59, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Colón, L.A.; Dash, P.K.; Morales-Vías, F.A.; Lebrón-Dávila, M.; Ferchmin, P.A.; Redell, J.B.; Maldonado-Martínez, G.; Vélez-Torres, W.I. 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice. J. Neuroinflam. 2021, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vega, M.N.; Sreerama, S.; Carrasquillo-Carrion, K.; Roche-Lima, A.; Corey Best, S.; Sugaya, K.; Ferchmin, P.A.; Eterovic, V.A.; Martins, A.H. 4R-Cembranoid treatment alters gene expression of RAW264.7 macrophages in basal and inflammatory conditions. Preprints 2020, 2020040278. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M.; Perrin, D.R.; Mandryk, M. Fungitoxicity of duvatrienediols associated with the cuticular wax of tobacco leaves. J. Phytopathol. 1977, 90, 243–249. [Google Scholar] [CrossRef]
- Menetrez, M.L.; Spurr, H.W.; Danehower, D.A.; Lawson, D.R. Influence of tobacco leaf surface chemicals on germination of Peronospora tabacina adam sporangia. J. Chem. Ecol. 1990, 16, 1565–1576. [Google Scholar] [CrossRef]
- Kennedy, B.S.; Nielsen, M.T.; Severson, R.F. Biorationals from Nicotiana protect cucumbers against Colletotrichum lagenarium (Pass.) ell. & halst disease development. J. Chem. Ecol. 1995, 21, 221–231. [Google Scholar]
- Aqil, F.; Zahin, M.; El-Sayed, K.A.; Ahmad, I.; Orabi, K.Y.; Arif, J.M. Antimicrobial, antioxidant, and antimutagenic activities of selected marine natural products and tobacco cembranoids. Drug Chem. Toxicol. 2011, 34, 167–179. [Google Scholar] [CrossRef]
- Duan, C.B.; Du, Y.; Hou, X.; Yan, N.; Dong, W.; Mao, X.; Zhang, Z. Chemical basis of the fungicidal activity of tobacco extracts against Valsa mali. Molecules 2016, 21, 1743. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Shi, J.; Xue, S.J.; Zhang, Z. Analyses of effects of α-cembratrien-diol on cell morphology and transcriptome of Valsa mali var. mali. Food Chem. 2017, 214, 110–118. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, P.; Xie, S.; Yuan, X.; Hou, X.; Yan, N.; Fang, Y.; Du, Y. In Vitro and in vivo antifungal activity and preliminary mechanism of cembratrien-diols against Botrytis cinerea. Ind. Crops Prod. 2020, 154, 112745. [Google Scholar] [CrossRef]
- Wang, J.; Xu, K.; Zhang, J.; Ren, G.; Yang, X.; Zhang, Z.; Zhang, Y.; Xiao, Y.; Du, Y. Systematic activity-oriented separation and structure-activity relationship of tobacco cembranoids. Ind. Crops Prod. 2021, 173, 114136. [Google Scholar] [CrossRef]
- Ferchmin, P.A.; Eterovic, V.A.; Maldonado, H.M. Neuronal Circuit-Dependent Neuroprotection by Interaction between Nicotinic Receptors. PCT Patent 2008/002594A2, 3 January 2008. [Google Scholar]
- Ford, B.D.; Ferchmin, P.A.; Eterovic, V.A. Methods and Compositions for Protecting and Treating Neuroinjury. PCT Patent 2011/008585, 20 January 2011. [Google Scholar]
- Ferchmin, P.A.; Eterovic, V.A.; Rodriguez, J.W.; Rios-Olivares, E.O.; Martins, A. Therapeutic Application of Cembranoids against HIV Virus Replication, HIV-Associated Neurocognitive Disorders and HIV Virus-Induced Inflammation. U.S. Patent 8835512B2, 16 September 2014. [Google Scholar]
- Eterovic, V.A.; Ferchmin, P.A.; Hann, R.M.; Pagan, O.R.; Rodriguez, A.D.; Rosario, O. Tobacco Cembranoids Block the Expression of the Behavioral Sensitization to Nicotine and Inhibit Neuronal Acetylcholine Receptors. U.S. Patent 6489357B1, 3 December 2002. [Google Scholar]
- El Sayed, K.A.; Ebrahim, H.Y.; Mohyeldin, M.M.; Hailat, M.M. Therapeutics and Methods to Treat Angiogenesis Related Pathologies. PCT Patent 2018/213824A2, 22 November 2018. [Google Scholar]
- Suzuki, M.; Izawa, T.; Ekimoto, H.; Takahashi, K.; Nakatani, T.; Fujii, A. Antineoplastic Agent. PCT Patent 86/02835, 22 May 1986. [Google Scholar]
- El Sayed, K.E.; Shah, G.; Sylvester, P. Anticancer Tobacco Cembranoids. U.S. Patents 79777384B1, 12 July 2011. [Google Scholar]
- Mizusaki, S.; Yoshida, D.; Saito, Y. Antitumor Agent. U.S. Patent 4701570, 20 October 1987. [Google Scholar]
- Bai, B.; Mao, D.B.; Yang, J.; Yang, P.F.; Wang, L.; Zhang, G.H.; Yang, J.; Wei, T.; Huang, S. Preparation Method and Application of Cebatrien-4-ol-6-Carboxylate. CN Patent 109970558A, 5 July 2019. [Google Scholar]
- Zhang, Z.F.; Du, Y.M.; Yan, P.Z.; He, X.F.; Hou, X.D.; Duan, S.Z.; Fu, Q.J.; Wang, A.H. Method for Extracting Ceberane Diterpenes from Tobacco Inflorescence. CN Patent 105001052B, 28 October 2015. [Google Scholar]








| Patent Title | Application Fields | Patent No. | Ref. |
|---|---|---|---|
| Neuronal circuit-dependent neuroprotection by interaction between nicotinic receptors | Neuroprotective | WO2008/002594A2 | [52] |
| Methods and compositions for protecting and treating neuroinjury | Neuroprotective | WO2011/008585 | [53] |
| Therapeutic application of cembranoids against HIV virus replication, HIV-associated neurocognitive disorders and HIV virus-induced inflammation | Neuroprotective | US8835512B2 | [54] |
| Tobacco cembranoids block the expression of the behavioral sensitization to nicotine and inhibit neuronal acetylcholine receptors | Neuroprotective | US6489357B1 | [55] |
| Therapeutics and methods to treat angiogenesis related pathologies | Anticancer | WO2018/213824A2 | [56] |
| Antineoplastic agent | Anticancer | WO86/02835 | [57] |
| Anticancer tobacco cembranoids | Anticancer | US79777384B1 | [58] |
| Antitumor agent | Anticancer | US4701570 | [59] |
| Preparation method and application of cebatrien-4-ol-6-carboxylate | Anticancer | CN109970558A | [60] |
| Method for extracting cembrane diterpenes from tobacco inflorescence | Antimicrobial | CN105001052A | [61] |
| NO. | Chemical Structures | Potent Bioactivities (IC50 or EC50 Value) | Ref. |
|---|---|---|---|
| α-CBD |  | Anti-proliferation on MDA-MB-231 (34.3 μM), MDA-MB-468 (39.3 μM), MCF-7 (61.2 μM), T-47D (61.9μM), SK-BR-3 (48.9 μM), and BT-474 (52.6 μM) cell lines; Antifungal effect against P. tabacina (24 μg/mL), V. mali (18.0 μg/mL), and B. cinerea (9.67–16.38 μg/mL); | [21,22,28,44,45] |
| β-CBD |  | Protective against neurotoxicity induced by NMDA (0.24 μM), POX (0.8 μM), and DFP in rat hippocampal slices; Protective activities on 6-OHDA-induced PD model; Antifungal effect against P. tabacina (17 μg/mL), V. mali, and B. cinerea (9.67–16.38 μg/mL); | [35,36,37,39,44,45] |
| 8 |  | Anti-proliferation on MDA-MB-231 (9.1 μM) and MDA-MB-468 cells (10.6 μM); Inhibition on c-Met catalytic activity (3.4 μM); | [21] |
| 12 |  | Anti-proliferation on MDA-MB-231 (5.3 μM) and MDA-MB-468 cells (6.6 μM); Inhibition on c-Met catalytic activity (2.9 μM); | [21] |
| 16 |  | Anti-proliferation on MDA-MB-231 (4.9 μM) and MDA-MB-468 cells (5.8 μM); Inhibition on c-Met catalytic activity (1.8 μM); | [21] |
| 18 |  | Anti-proliferation on MDA-MB-231 (1.3 μM), MDA-MB-468 (2.1 μM), MCF-7 (7.2 μM), T-47D (11.2 μM), SK-BR-3 (4.7 μM), and BT-474 (3.9 μM) cell lines; Inhibition on c-Met catalytic activity (1.1 μM); | [21,22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Du, X.; Ren, X.; Li, X.; Li, H.; Fu, X.; Wei, X. Structural Modifications and Biological Activities of Natural α- and β-Cembrenediol: A Comprehensive Review. Pharmaceuticals 2022, 15, 601. https://doi.org/10.3390/ph15050601
Xu K, Du X, Ren X, Li X, Li H, Fu X, Wei X. Structural Modifications and Biological Activities of Natural α- and β-Cembrenediol: A Comprehensive Review. Pharmaceuticals. 2022; 15(5):601. https://doi.org/10.3390/ph15050601
Chicago/Turabian StyleXu, Kuo, Xinying Du, Xia Ren, XiuXue Li, Hui Li, Xianjun Fu, and Xiaoyi Wei. 2022. "Structural Modifications and Biological Activities of Natural α- and β-Cembrenediol: A Comprehensive Review" Pharmaceuticals 15, no. 5: 601. https://doi.org/10.3390/ph15050601