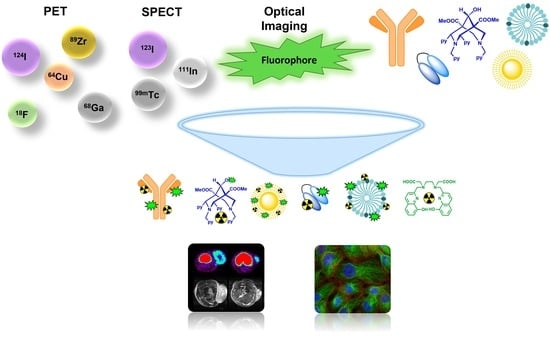
Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives
Abstract
:1. Introduction
2. Fluorescence Imaging for Biomedical Applications
2.1. NIR Metal Complex Imaging Agents
2.1.1. Lanthanide-Based Molecules
2.1.2. Non-Lanthanide-Based Molecules
3. Modular Ligand Systems
3.1. Organic-Based Systems
3.2. Metal-Based Systems
3.3. Mixed Ligand Systems
4. Nanoscale Systems
4.1. Organic Nanoscale Systems
4.2. Inorganic Nanoscale Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achilefu, S. Introduction to concepts and strategies for molecular imaging. Chem. Rev. 2010, 110, 2575–2578. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, P.J.; Radda, G.K. Molecular imaging perspectives. J. R. Soc. Interface 2005, 2, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Sedlacek, O.; Nadar, R.A.; Hruby, M.; Stephan, H. Radiolabelled Polymeric Materials for Imaging and Treatment of Cancer: Quo Vadis? Adv. Healthc. Mater. 2017, 6, 31. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef]
- Qaim, S.M. Theranostic radionuclides: Recent advances in production methodologies. J. Radioanal. Nucl. Chem. 2019, 322, 1257–1266. [Google Scholar] [CrossRef]
- Eckerman, K.; Endo, A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pu, K. Molecular Probes for Autofluorescence-Free Optical Imaging. Chem. Rev. 2021, 121, 13086–13131. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Mu, X.; Zhang, X.D.; Ming, D. The Near-Infrared-II Fluorophores and Advanced Microscopy Technologies Development and Application in Bioimaging. Bioconj. Chem. 2020, 31, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, M.; Jang, M.; Choi, Y.; Choi, W.; Kang, S.; Choi, W. Deep optical imaging within complex scattering media. Nat. Rev. Phys. 2020, 2, 141–158. [Google Scholar] [CrossRef] [Green Version]
- Juzenas, P.; Juzeniene, A.; Kaalhus, O.; Iani, V.; Moan, J. Noninvasive fluorescence excitation spectroscopy during application of 5-aminolevulinic acid in vivo. Photochem. Photobiol. Sci. 2002, 1, 745–748. [Google Scholar] [CrossRef]
- Cheon, J.; Lee, J.H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef]
- Lu, F.M.; Yuan, Z. PET/SPECT molecular imaging in clinical neuroscience: Recent advances in the investigation of CNS diseases. Quant. Imaging Med. Surg. 2015, 5, 433–447. [Google Scholar] [CrossRef]
- Massoud, T.F.; Gambhir, S.S. Molecular imaging in living subjects: Seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Faizi, M.S.H.; Rather, J.A.; Khan, M.S. Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: A review. Bioorgan. Med. Chem. 2017, 25, 2017–2034. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, W.H.; Tian, J.; Cheng, Z. NIRF Nanoprobes for Cancer Molecular Imaging: Approaching Clinic. Trends Mol. Med. 2020, 26, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dyes Pigm. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Reja, S.I.; Minoshima, M.; Hori, Y.; Kikuchi, K. Near-infrared fluorescent probes: A next-generation tool for protein-labeling applications. Chem. Sci. 2021, 12, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, S.; Yang, N.-D.; Zhang, C.; Wu, Q.; Yu, C. Recent development of small-molecule organic fluorophores for multifunctional bioimaging in the second near-infrared window. J. Lumin. 2020, 225, 117338. [Google Scholar] [CrossRef]
- Qu, C.; Xiao, Y.; Zhou, H.; Ding, B.; Li, A.; Lin, J.; Zeng, X.; Chen, H.; Qian, K.; Zhang, X.; et al. Quaternary Ammonium Salt Based NIR-II Probes for In Vivo Imaging. Adv. Opt. Mater. 2019, 7, 1900229. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Yang, Y.; Yang, Y.; Zhang, K.; Guo, L.; Ge, H.; Chen, X.; Liu, J.; Feng, H. Molecular Engineering of an Organic NIR-II Fluorophore with Aggregation-Induced Emission Characteristics for In Vivo Imaging. Small 2019, 15, 1805549. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Li, Z.; Zhai, S.; Lv, W.; Liu, Z. Lighting Up NIR-II Fluorescence in Vivo: An Activable Probe for Noninvasive Hydroxyl Radical Imaging. Anal. Chem. 2019, 91, 15757–15762. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel aza-BODIPY based small molecular NIR-II fluorophores for in vivo imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef]
- Ariztia, J.; Solmont, K.; Moïse, N.P.; Specklin, S.; Heck, M.P.; Lamandé-Langle, S.; Kuhnast, B. PET/Fluorescence Imaging: An Overview of the Chemical Strategies to Build Dual Imaging Tools. Bioconjug. Chem. 2022, 33, 24–52. [Google Scholar] [CrossRef]
- Azhdarinia, A.; Ghosh, P.; Ghosh, S.; Wilganowski, N.; Sevick-Muraca, E.M. Dual-Labeling Strategies for Nuclear and Fluorescence Molecular Imaging: A Review and Analysis. Mol. Imaging Biol. 2012, 14, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, B.P.; Wang, T.D. Exogenous Molecular Probes for Targeted Imaging in Cancer: Focus on Multi-modal Imaging. Cancers 2010, 2, 1251–1287. [Google Scholar] [CrossRef] [PubMed]
- Kuil, J.; Velders, A.H.; van Leeuwen, F.W. Multimodal tumor-targeting peptides functionalized with both a radio- and a fluorescent label. Bioconjug. Chem 2010, 21, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, X. Dual-modality probes for in vivo molecular imaging. Mol. Imaging 2009, 8, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibold, U.; Wangler, B.; Schirrmacher, R.; Wangler, C. Bimodal imaging probes for combined PET and OI: Recent developments and future directions for hybrid agent development. Biomed. Res. Int. 2014, 2014, 153741. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Gott, M.D.; Pietzsch, H.J.; Stephan, H. Nuclear and optical dual-labelled imaging agents. Nuklearmedizin 2016, 55, 41–50. [Google Scholar] [CrossRef]
- Vaz, S.C.; Oliveira, F.; Herrmann, K.; Veit-Haibach, P. Nuclear medicine and molecular imaging advances in the 21st century. Br. J. Radiol. 2020, 93, 20200095. [Google Scholar] [CrossRef]
- Bünzli, J.C. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Thibon, A.; Pierre, V.C. Principles of responsive lanthanide-based luminescent probes for cellular imaging. Anal. Bioanal. Chem. 2009, 394, 107–120. [Google Scholar] [CrossRef]
- Weissman, S.I. Intramolecular Energy Transfer The Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Cable, M.L.; Levine, D.J.; Kirby, J.P.; Gray, H.B.; Ponce, A. Luminescent lanthanide sensors. Adv. Inorg. Chem. 2011, 63, 1–45. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and Kinetic Data for Macrocycle Interaction with Cations, Anions, and Neutral Molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Bodman, S.E.; Butler, S.J. Advances in anion binding and sensing using luminescent lanthanide complexes. Chem. Sci. 2021, 12, 2716–2734. [Google Scholar] [CrossRef]
- New, E.J.; Parker, D.; Smith, D.G.; Walton, J.W. Development of responsive lanthanide probes for cellular applications. Curr. Opin. Chem. Biol. 2010, 14, 238–246. [Google Scholar] [CrossRef]
- D’Aléo, A.; Andraud, C.; Maury, O. Two-photon Absorption of Lanthanide Complexes: From Fundamental Aspects to Biphotonic Imaging Applications. In Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials; Wiley: Hoboken, NJ, USA, 2014; pp. 197–230. [Google Scholar]
- Bui, A.T.; Beyler, M.; Grichine, A.; Duperray, A.; Mulatier, J.-C.; Guyot, Y.; Andraud, C.; Tripier, R.; Brasselet, S.; Maury, O. Near infrared two photon imaging using a bright cationic Yb(iii) bioprobe spontaneously internalized into live cells. Chem. Commun. 2017, 53, 6005–6008. [Google Scholar] [CrossRef]
- Ning, Y.; Chen, S.; Chen, H.; Wang, J.-X.; He, S.; Liu, Y.-W.; Cheng, Z.; Zhang, J.-L. A proof-of-concept application of water-soluble ytterbium(iii) molecular probes in in vivo NIR-II whole body bioimaging. Inorg. Chem. Front. 2019, 6, 1962–1967. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, Y.-W.; Yang, Z.-S.; Yao, Y.; Kang, L.; Sessler, J.L.; Zhang, J.-L. Split and Use: Structural Isomers for Diagnosis and Therapy. J. Am. Chem. Soc. 2020, 142, 6761–6768. [Google Scholar] [CrossRef]
- Ning, Y.; Tang, J.; Liu, Y.-W.; Jing, J.; Sun, Y.; Zhang, J.-L. Highly luminescent, biocompatible ytterbium(iii) complexes as near-infrared fluorophores for living cell imaging. Chem. Sci. 2018, 9, 3742–3753. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Zhang, H.; Ran, G.; Mangel, D.N.; Yao, Y.; Zhang, R.; Tan, J.; Zhang, W.; Song, J.; Sessler, J.L.; et al. Metal Modulation: An Easy-to-Implement Tactic for Tuning Lanthanide Phototheranostics. J. Am. Chem. Soc. 2021, 143, 7541–7552. [Google Scholar] [CrossRef]
- Hamon, N.; Roux, A.; Beyler, M.; Mulatier, J.-C.; Andraud, C.; Nguyen, C.; Maynadier, M.; Bettache, N.; Duperray, A.; Grichine, A.; et al. Pyclen-Based Ln(III) Complexes as Highly Luminescent Bioprobes for In Vitro and In Vivo One- and Two-Photon Bioimaging Applications. J. Am. Chem. Soc. 2020, 142, 10184–10197. [Google Scholar] [CrossRef] [PubMed]
- Nizou, G.; Favaretto, C.; Borgna, F.; Grundler, P.V.; Saffon-Merceron, N.; Platas-Iglesias, C.; Fougère, O.; Rousseaux, O.; van der Meulen, N.P.; Müller, C.; et al. Expanding the Scope of Pyclen-Picolinate Lanthanide Chelates to Potential Theranostic Applications. Inorg. Chem. 2020, 59, 11736–11748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, X.; Chau, H.-F.; Thor, W.; Jiang, L.; Zha, S.; Fok, W.-Y.; Mak, H.-N.; Zhang, J.; Cai, J.; et al. Lanthanide–Cyclen–Camptothecin Nanocomposites for Cancer Theranostics Guided by Near-Infrared and Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2021, 4, 271–278. [Google Scholar] [CrossRef]
- Martin, K.E.; Cosby, A.G.; Boros, E. Multiplex and In Vivo Optical Imaging of Discrete Luminescent Lanthanide Complexes Enabled by In Situ Cherenkov Radiation Mediated Energy Transfer. J. Am. Chem. Soc. 2021, 143, 9206–9214. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Zhang, K.Y.; Liu, S.; Zhao, Q. Phosphorescent iridium(III) complexes as lifetime-based biological sensors for photoluminescence lifetime imaging microscopy. Coord. Chem. Rev. 2022, 453, 214334. [Google Scholar] [CrossRef]
- Yang, Q.; Jin, H.; Gao, Y.; Lin, J.; Yang, H.; Yang, S. Photostable Iridium(III)–Cyanine Complex Nanoparticles for Photoacoustic Imaging Guided Near-Infrared Photodynamic Therapy in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 15417–15425. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Fang, L.; Gao, C.; Xu, C.; Gou, S. Iridium(III) Complex–Derived Polymeric Micelles with Low Dark Toxicity and Strong NIR Excitation for Phototherapy and Chemotherapy. Small 2020, 16, 2000363. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, K.; Xu, G.; Liu, X.; Zhao, Q.; Xu, C.; Gou, S. An Iridium (III) Complex Bearing a Donor–Acceptor–Donor Type Ligand for NIR-Triggered Dual Phototherapy. Adv. Funct. Mater. 2021, 31, 2008325. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Wu, J.; Luo, Z.; Zhou, W.; Li, A.; Chen, Y.; Rouzi, T.; Tian, T.; Zhou, H.; et al. All-in-one mitochondria-targeted NIR-II fluorophores for cancer therapy and imaging. Chem. Sci. 2021, 12, 1843–1850. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Gu, M.; Lu, D.; Xiong, X.; Zhang, Z.; Pan, Y.; Liao, Y.; Ding, Q.; Gong, W.; et al. A Second Near-Infrared Ru(II) Polypyridyl Complex for Synergistic Chemo-Photothermal Therapy. J. Med. Chem. 2022, 65, 2225–2237. [Google Scholar] [CrossRef]
- An, F.; Nurili, F.; Sayman, H.; Ozer, Z.; Cakiroglu, H.; Aras, O.; Ting, R. One-Step, Rapid, 18F–19F Isotopic Exchange Radiolabeling of Difluoro-dioxaborinins: Substituent Effect on Stability and In Vivo Applications. J. Med. Chem. 2020, 63, 12693–12706. [Google Scholar] [CrossRef] [PubMed]
- An, F.-F.; Kommidi, H.; Chen, N.; Ting, R. A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. Int. J. Mol. Sci. 2017, 18, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kim, K.; Son, S.-H.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T.; Byun, Y.; Choe, Y.S. 18F-Labeled BODIPY Dye: A Potential Prosthetic Group for Brain Hybrid PET/Optical Imaging Agents. ACS Chem. Neurosci. 2019, 10, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Kreimerman, I.; Mora-Ramirez, E.; Reyes, L.; Bardiès, M.; Savio, E.; Engler, H. Dosimetry and Toxicity Studies of the Novel Sulfonamide Derivative of Sulforhodamine 101([18F]SRF101) at a Preclinical Level. Curr. Radiopharm. 2019, 12, 40–48. [Google Scholar] [CrossRef]
- Kang, N.-Y.; Lee, J.Y.; Lee, S.H.; Song, I.H.; Hwang, Y.H.; Kim, M.J.; Phue, W.H.; Agrawalla, B.K.; Wan, S.Y.D.; Lalic, J.; et al. Multimodal Imaging Probe Development for Pancreatic β Cells: From Fluorescence to PET. J. Am. Chem. Soc. 2020, 142, 3430–3439. [Google Scholar] [CrossRef]
- Schwegmann, K.; Hohn, M.; Hermann, S.; Schäfers, M.; Riemann, B.; Haufe, G.; Wagner, S.; Breyholz, H.-J. Optimizing the Biodistribution of Radiofluorinated Barbiturate Tracers for Matrix Metalloproteinase Imaging by Introduction of Fluorescent Dyes as Pharmacokinetic Modulators. Bioconjug. Chem. 2020, 31, 1117–1132. [Google Scholar] [CrossRef]
- Kanagasundaram, T.; Laube, M.; Wodtke, J.; Kramer, C.S.; Stadlbauer, S.; Pietzsch, J.; Kopka, K. Radiolabeled Silicon-Rhodamines as Bimodal PET/SPECT-NIR Imaging Agents. Pharmaceuticals 2021, 14, 1155. [Google Scholar] [CrossRef]
- Wang, W.; Ke, S.; Kwon, S.; Yallampalli, S.; Cameron, A.G.; Adams, K.E.; Mawad, M.E.; Sevick-Muraca, E.M. A New Optical and Nuclear Dual-Labeled Imaging Agent Targeting Interleukin 11 Receptor Alpha-Chain. Bioconjug. Chem. 2007, 18, 397–402. [Google Scholar] [CrossRef]
- Tiannv, L.; Jin, S.; Yao, H.; Min, Y.; Haibin, S.; Lijun, T. Near-infrared fluorescent labeled CGRRAGGSC peptides for optical imaging of IL-11Rα in athymic mice bearing tumor xenografts. J. Biomed. Res. 2019, 33, 391. [Google Scholar] [CrossRef]
- Derks, Y.H.W.; Rijpkema, M.; Amatdjais-Groenen, H.I.V.; Loeff, C.C.; de Roode, K.E.; Kip, A.; Laverman, P.; Lütje, S.; Heskamp, S.; Löwik, D.W.P.M. Strain-Promoted Azide–Alkyne Cycloaddition-Based PSMA-Targeting Ligands for Multimodal Intraoperative Tumor Detection of Prostate Cancer. Bioconjug. Chem. 2022, 33, 194–205. [Google Scholar] [CrossRef]
- Boulay, A.; Artigau, M.; Coulais, Y.; Picard, C.; Mestre-Voegtle, B.; Benoist, E. First dinuclear Re/Tc complex as a potential bimodal Optical/SPECT molecular imaging agent. Dalton Trans. 2011, 40, 6206–6209. [Google Scholar] [CrossRef] [PubMed]
- François, A.; Auzanneau, C.; Le Morvan, V.; Galaup, C.; Godfrey, H.S.; Marty, L.; Boulay, A.; Artigau, M.; Mestre-Voegtlé, B.; Leygue, N.; et al. A functionalized heterobimetallic 99mTc/Re complex as a potential dual-modality imaging probe: Synthesis, photophysical properties, cytotoxicity and cellular imaging investigations. Dalton Trans. 2014, 43, 439–450. [Google Scholar] [CrossRef]
- Yazdani, A.; Janzen, N.; Banevicius, L.; Czorny, S.; Valliant, J.F. Imidazole-based [2+1] Re(I)/99mTc(I) complexes as isostructural nuclear and optical probes. Inorg. Chem. 2015, 54, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Zhou, J.-W.; Chan, C.-F.; Lau, T.C.-K.; Kwong, D.W.J.; Tam, H.-L.; Mak, N.-K.; Wong, K.-L.; Wong, W.-K. Comparative Studies of the Cellular Uptake, Subcellular Localization, and Cytotoxic and Phototoxic Antitumor Properties of Ruthenium(II)–Porphyrin Conjugates with Different Linkers. Bioconjug. Chem. 2012, 23, 1623–1638. [Google Scholar] [CrossRef] [PubMed]
- Day, A.H.; Domarkas, J.; Nigam, S.; Renard, I.; Cawthorne, C.; Burke, B.P.; Bahra, G.S.; Oyston, P.C.F.; Fallis, I.A.; Archibald, S.J.; et al. Towards dual SPECT/optical bioimaging with a mitochondrial targeting, 99mTc(i) radiolabelled 1,8-naphthalimide conjugate. Dalton Trans. 2020, 49, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.C.; Ghosh, P.; Wilganowski, N.; Robinson, H.; Hall, M.A.; Dickinson, G.; Pinkston, K.L.; Harvey, B.R.; Sevick-Muraca, E.M.; Azhdarinia, A. Multimodal Chelation Platform for Near-Infrared Fluorescence/Nuclear Imaging. J. Med. Chem. 2013, 56, 406–416. [Google Scholar] [CrossRef]
- Ananias, H.J.K.; Yu, Z.; Dierckx, R.A.; van der Wiele, C.; Helfrich, W.; Wang, F.; Yan, Y.; Chen, X.; de Jong, I.J.; Elsinga, P.H. 99mTechnetium-HYNIC(tricine/TPPTS)-Aca-Bombesin(7–14) as a Targeted Imaging Agent with MicroSPECT in a PC-3 Prostate Cancer Xenograft Model. Mol. Pharm. 2011, 8, 1165–1173. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chi, C.; Xiao, X.; Wang, J.; Lang, L.; Ali, I.; Niu, G.; Zhang, L.; Tian, J.; et al. First-in-human study of PET and optical dual-modality image-guided surgery in glioblastoma using 68Ga-IRDye800CW-BBN. Theranostics 2018, 8, 2508–2520. [Google Scholar] [CrossRef]
- Wang, X.; Jaraquemada-Peláez, M.d.G.; Cao, Y.; Pan, J.; Lin, K.-S.; Patrick, B.O.; Orvig, C. H2hox: Dual-Channel Oxine-Derived Acyclic Chelating Ligand for 68Ga Radiopharmaceuticals. Inorg. Chem. 2019, 58, 2275–2285. [Google Scholar] [CrossRef]
- Baranski, A.-C.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendörfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11–Derived Dual-Labeled PSMA Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Eder, A.-C.; Omrane, M.A.; Stadlbauer, S.; Roscher, M.; Khoder, W.Y.; Gratzke, C.; Kopka, K.; Eder, M.; Meyer, P.T.; Jilg, C.A.; et al. The PSMA-11-derived hybrid molecule PSMA-914 specifically identifies prostate cancer by preoperative PET/CT and intraoperative fluorescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, D.; Cornelissen, B. Emerging chelators for nuclear imaging. Curr. Opin. Chem. Biol. 2021, 63, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.A.; Kwon, S.; Robinson, H.; Lachance, P.A.; Azhdarinia, A.; Ranganathan, R.; Price, R.E.; Chan, W.; Sevick-Muraca, E.M. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate 2012, 72, 129–146. [Google Scholar] [CrossRef]
- Paudyal, P.; Paudyal, B.; Iida, Y.; Oriuchi, N.; Hanaoka, H.; Tominaga, H.; Ishikita, T.; Yoshioka, H.; Higuchi, T.; Endo, K. Dual functional molecular imaging probe targeting CD20 with PET and optical imaging. Oncol. Rep. 2009, 22, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Sampath, L.; Kwon, S.; Hall, M.A.; Price, R.E.; Sevick-Muraca, E.M. Detection of Cancer Metastases with a Dual-labeled Near-Infrared/Positron Emission Tomography Imaging Agent. Trans. Oncol. 2010, 3, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Wang, H.; Wang, M.; Li, Z.; Wu, Z. Synthesis and Evaluation of 64Cu-DOTA-NT-Cy5.5 as a Dual-Modality PET/Fluorescence Probe to Image Neurotensin Receptor-Positive Tumor. Mol. Pharm. 2015, 12, 3054–3061. [Google Scholar] [CrossRef] [Green Version]
- Edwards, W.B.; Xu, B.; Akers, W.; Cheney, P.P.; Liang, K.; Rogers, B.E.; Anderson, C.J.; Achilefu, S. Agonist-antagonist dilemma in molecular imaging: Evaluation of a monomolecular multimodal imaging agent for the somatostatin receptor. Bioconjug. Chem. 2008, 19, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Rodenberg, E.; Azhdarinia, A.; Lazard, Z.W.; Hall, M.; Kwon, S.K.; Wilganowski, N.; Salisbury, E.A.; Merched-Sauvage, M.; Olmsted-Davis, E.A.; Sevick-Muraca, E.M.; et al. Matrix metalloproteinase-9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng. Part A 2011, 17, 2487–2496. [Google Scholar] [CrossRef]
- Sargeson, A.M. The potential for the cage complexes in biology. Coord. Chem. Rev. 1996, 151, 89–114. [Google Scholar] [CrossRef]
- Joshi, T.; Kubeil, M.; Nsubuga, A.; Singh, G.; Gasser, G.; Stephan, H. Harnessing the Coordination Chemistry of 1,4,7-Triazacyclononane for Biomimicry and Radiopharmaceutical Applications. ChemPlusChem 2018, 83, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Abdel-Atti, D.; Zhang, Y.; Carlin, S.; Clardy, S.M.; Keliher, E.J.; Weber, W.A.; Lewis, J.S.; Reiner, T. In Vivo Imaging of GLP-1R with a Targeted Bimodal PET/Fluorescence Imaging Agent. Bioconjug. Chem. 2014, 25, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G.; Tjioe, L.; Graham, B.; Belousoff, M.J.; Juran, S.; Walther, M.; Kunstler, J.U.; Bergmann, R.; Stephan, H.; Spiccia, L. Synthesis, Copper(II) Complexation, 64Cu-Labeling, and Bioconjugation of a New Bis(2-pyridylmethyl) Derivative of 1,4,7-Triazacyclononane. Bioconjug. Chem. 2008, 19, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Viehweger, K.; Barbaro, L.; Garcia, K.P.; Joshi, T.; Geipel, G.; Steinbach, J.; Stephan, H.; Spiccia, L.; Graham, B. EGF receptor-targeting peptide conjugate incorporating a near-IR fluorescent dye and a novel 1,4,7-triazacyclononane-based 64Cu(II) chelator assembled via click chemistry. Bioconjug. Chem. 2014, 25, 1011–1022. [Google Scholar] [CrossRef]
- Bleiholder, C.; Börzel, H.; Comba, P.; Ferrari, R.; Heydt, M.; Kerscher, M.; Kuwata, S.; Laurenczy, G.; Lawrance, G.A.; Lienke, A.; et al. Coordination Chemistry of a New Rigid, Hexadentate Bispidine-Based Bis(amine)tetrakis(pyridine) Ligand. Inorg. Chem. 2005, 44, 8145–8155. [Google Scholar] [CrossRef]
- Comba, P.; Kerscher, M.; Rück, K.; Starke, M. Bispidines for radiopharmaceuticals. Dalton Trans. 2018, 47, 9202–9220. [Google Scholar] [CrossRef]
- Juran, S.; Walther, M.; Stephan, H.; Bergmann, R.; Steinbach, J.; Kraus, W.; Emmerling, F.; Comba, P. Hexadentate bispidine derivatives as versatile bifunctional chelate agents for copper(II) radioisotopes. Bioconjug. Chem. 2009, 20, 347–359. [Google Scholar] [CrossRef]
- Singh, G.; Zarschler, K.; Hunoldt, S.; Martínez, I.I.S.; Ruehl, C.L.; Matterna, M.; Bergmann, R.; Máthé, D.; Hegedüs, N.; Bachmann, M.; et al. Versatile Bispidine-Based Bifunctional Chelators for 64CuII-Labelling of Biomolecules. Chem. Eur. J. 2020, 26, 1989–2001. [Google Scholar] [CrossRef]
- Stephan, H.; Walther, M.; Fahnemann, S.; Ceroni, P.; Molloy, J.K.; Bergamini, G.; Heisig, F.; Muller, C.E.; Kraus, W.; Comba, P. Bispidines for dual imaging. Chem. Eur. J. 2014, 20, 17011–17018. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Guo, K.; Teng, B.; Edwards, W.B.; Anderson, C.J.; Vasalatiy, O.; Gandjbakhche, A.; Griffiths, G.L.; Achilefu, S. Radioactivity-Synchronized Fluorescence Enhancement Using a Radionuclide Fluorescence-Quenched Dye. J. Am. Chem. Soc. 2009, 131, 9198–9200. [Google Scholar] [CrossRef] [Green Version]
- Abad-Galán, L.; Cieslik, P.; Comba, P.; Gast, M.; Maury, O.; Neupert, L.; Roux, A.; Wadepohl, H. Excited State Properties of Lanthanide(III) Complexes with a Nonadentate Bispidine Ligand. Chem. Eur. J. 2021, 27, 10303–10312. [Google Scholar] [CrossRef] [PubMed]
- Bruchertseifer, F.; Comba, P.; Martin, B.; Morgenstern, A.; Notni, J.; Starke, M.; Wadepohl, H. First-Generation Bispidine Chelators for 213BiIII Radiopharmaceutical Applications. ChemMedChem 2020, 15, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Meimetis, L.G.; Boros, E.; Carlson, J.C.; Ran, C.; Caravan, P.; Weissleder, R. Bioorthogonal Fluorophore Linked DFO—Technology Enabling Facile Chelator Quantification and Multimodal Imaging of Antibodies. Bioconjug. Chem. 2016, 27, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.K.; Zettlitz, K.A.; Tavaré, R.; Kobayashi, N.; Reiter, R.E.; Wu, A.M. Dual-Modality ImmunoPET/Fluorescence Imaging of Prostate Cancer with an Anti-PSCA Cys-Minibody. Theranostics 2018, 8, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Sarparanta, M.; Pourat, J.; Carnazza, K.E.; Tang, J.; Paknejad, N.; Reiner, T.; Kostiainen, M.A.; Lewis, J.S. Multimodality labeling strategies for the investigation of nanocrystalline cellulose biodistribution in a mouse model of breast cancer. Nucl. Med. Biol. 2020, 80–81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, W.; Barnhart, T.E.; Jiang, D.; Cao, T.; Fan, K.; Engle, J.W.; Liu, J.; Chen, W.; Cai, W. ImmunoPET/NIRF/Cerenkov multimodality imaging of ICAM-1 in pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2737–2748. [Google Scholar] [CrossRef]
- Brandt, M.; Cowell, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. Radiolabelling of the octadentate chelators DFO* and oxoDFO* with zirconium-89 and gallium-68. J. Biol. Inorg. Chem. 2020, 25, 789–796. [Google Scholar] [CrossRef]
- Vugts, D.J.; Klaver, C.; Sewing, C.; Poot, A.J.; Adamzek, K.; Huegli, S.; Mari, C.; Visser, G.W.M.; Valverde, I.E.; Gasser, G.; et al. Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Comba, P.; Jermilova, U.; Orvig, C.; Patrick, B.O.; Ramogida, C.F.; Rück, K.; Schneider, C.; Starke, M. Octadentate Picolinic Acid-Based Bispidine Ligand for Radiometal Ions. Chem. Eur. J. 2017, 23, 15945–15956. [Google Scholar] [CrossRef]
- Wang, X.; Jaraquemada-Peláez, M.d.G.; Rodríguez-Rodríguez, C.; Cao, Y.; Buchwalder, C.; Choudhary, N.; Jermilova, U.; Ramogida, C.F.; Saatchi, K.; Häfeli, U.O.; et al. H4octox: Versatile Bimodal Octadentate Acyclic Chelating Ligand for Medicinal Inorganic Chemistry. J. Am. Chem. Soc. 2018, 140, 15487–15500. [Google Scholar] [CrossRef]
- Southcott, L.; Wang, X.; Choudhary, N.; Wharton, L.; Patrick, B.O.; Yang, H.; Zarschler, K.; Kubeil, M.; Stephan, H.; Jaraquemada-Peláez, M.d.G.; et al. H2pyhox—Octadentate Bis(pyridyloxine). Inorg. Chem. 2021, 60, 12186–12196. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Barrett, K.E.; Kubeil, M.; Radchenko, V.; Engle, J.W.; Stephan, H.; Jaraquemada-Peláez, M.d.G.; Orvig, C. Metal ion size profoundly affects H3glyox chelate chemistry. RSC Adv. 2021, 11, 15663–15674. [Google Scholar] [CrossRef]
- Choudhary, N.; Guadalupe Jaraquemada-Peláez, M.D.; Zarschler, K.; Wang, X.; Radchenko, V.; Kubeil, M.; Stephan, H.; Orvig, C. Chelation in One Fell Swoop: Optimizing Ligands for Smaller Radiometal Ions. Inorg. Chem. 2020, 59, 5728–5741. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, T.I.; Wang, X.; Southcott, L.; Wagner, H.K.; Kubeil, M.; Stephan, H.; Jaraquemada-Peláez, M.d.G.; Orvig, C. Rapid Thermodynamically Stable Complex Formation of [nat/111In]In3+, [nat/90Y]Y3+, and [nat/177Lu]Lu3+ with H6dappa. Inorg. Chem. 2020, 59, 7238–7251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jaraquemada-Peláez, M.d.G.; Kuo, H.-T.; Merkens, H.; Choudhary, N.; Gitschtaler, K.; Jermilova, U.; Colpo, N.; Uribe-Munoz, C.; Radchenko, V.; et al. Functionally Versatile and Highly Stable Chelator for 111In and 177Lu: Proof-of-Principle Prostate-Specific Membrane Antigen Targeting. Bioconjug. Chem. 2019, 30, 1539–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Jaraquemada-Peláez, M.d.G.; Aluicio-Sarduy, E.; Wang, X.; Jiang, D.; Sakheie, M.; Kuo, H.-T.; Barnhart, T.E.; Cai, W.; Radchenko, V.; et al. [nat/44Sc(pypa)]−: Thermodynamic Stability, Radiolabeling, and Biodistribution of a Prostate-Specific-Membrane-Antigen-Targeting Conjugate. Inorg. Chem. 2020, 59, 1985–1995. [Google Scholar] [CrossRef]
- Southcott, L.; Li, L.; Patrick, B.O.; Stephan, H.; Jaraquemada-Peláez, M.d.G.; Orvig, C. [nat/89Zr][Zr(pypa)]: Thermodynamically Stable and Kinetically Inert Binary Nonadentate Complex for Radiopharmaceutical Applications. Inorg. Chem. 2021, 60, 18082–18093. [Google Scholar] [CrossRef]
- Chen, G.Y.; Roy, I.; Yang, C.H.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.B.; Liu, C.H. Big Potential from Small Agents: Nanoparticles for Imaging-Based Companion Diagnostics. ACS Nano 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Elsabahy, M.; Heo, G.S.; Lim, S.M.; Sun, G.R.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Chen, H.; Irfan, M.; Bajwa, S.Z.; Khan, W.S.; Baig, S.M.; Dai, Z.F. Fluorescence Guided Sentinel Lymph Node Mapping: From Current Molecular Probes to Future Multimodal Nanoprobes. Bioconjug. Chem. 2019, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Cai, W.B.; Chen, X.Y. Positron Emission Tomography Imaging Using Radio labeled Inorganic Nanomaterials. Acc. Chem. Res. 2015, 48, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, D.L.; Ehlerding, E.B.; Cai, W.B. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. 2019, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Feldmann, A.; Koristka, S.; Schäfer, M.; Bergmann, R.; Mitwasi, N.; Berndt, N.; Bachmann, D.; Kegler, A.; Schmitz, M.; et al. A theranostic PSMA ligand for PET imaging and retargeting of T cells expressing the universal chimeric antigen receptor UniCAR. Oncoimmunology 2019, 8, 1659095. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, A.; Arndt, C.; Töpfer, K.; Stamova, S.; Krone, F.; Cartellieri, M.; Koristka, S.; Michalk, I.; Lindemann, D.; Schmitz, M.; et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012, 189, 3249–3259. [Google Scholar] [CrossRef] [Green Version]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomedicine 2016, 12, 1663–1701. [Google Scholar] [CrossRef]
- Blanco, V.M.; Chu, Z.T.; LaSance, K.; Gray, B.D.; Pak, K.Y.; Rider, T.; Greis, K.D.; Qi, X.Y. Optical and nuclear imaging of glioblastoma with phosphatidylserine-targeted nanovesicles. Oncotarget 2016, 7, 32866–32875. [Google Scholar] [CrossRef]
- Jung, K.O.; Kim, Y.-H.; Chung, S.-J.; Lee, C.-H.; Rhee, S.; Pratx, G.; Chung, J.-K.; Youn, H. Identification of Lymphatic and Hematogenous Routes of Rapidly Labeled Radioactive and Fluorescent Exosomes through Highly Sensitive Multimodal Imaging. Int. J. Mol. Sci. 2020, 21, 7850. [Google Scholar] [CrossRef]
- Persico, M.G.; Marenco, M.; De Matteis, G.; Manfrinato, G.; Cavenaghi, G.; Sgarella, A.; Aprile, C.; Lodola, L. 99mTc-68Ga-ICG-Labelled Macroaggregates and Nanocolloids of Human Serum Albumin: Synthesis Procedures of a Trimodal Imaging Agent Using Commercial Kits. Contrast Media Mol. Imaging 2020, 2020, 3629705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, I.; Mattingly, S.; Wuest, M.; Leier, S.; Vakili, M.R.; Weinfeld, M.; Lavasanifar, A.; Wuest, F. Synthesis and Analysis of 64Cu-Labeled GE11-Modified Polymeric Micellar Nanoparticles for EGFR-Targeted Molecular Imaging in a Colorectal Cancer Model. Mol. Pharm. 2020, 17, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Goel, S.; Liu, H.-J.; Carter, K.A.; Jiang, D.; Geng, J.; Kutyreff, C.J.; Engle, J.W.; Huang, W.-C.; Shao, S.; et al. Intrabilayer 64Cu Labeling of Photoactivatable, Doxorubicin-Loaded Stealth Liposomes. ACS Nano 2017, 11, 12482–12491. [Google Scholar] [CrossRef]
- Record, M.; Subra, C.; Silvente-Poirot, S.; Poirot, M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011, 81, 1171–1182. [Google Scholar] [CrossRef] [Green Version]
- van den Boorn, J.G.; Schlee, M.; Coch, C.; Hartmann, G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011, 29, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Neuber, C.; Zarschler, K.; Wodtke, J.; Meister, S.; Haag, R.; Pietzsch, J.; Stephan, H. Active Targeting of Dendritic Polyglycerols for Diagnostic Cancer Imaging. Small 2020, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- van Onzen, A.; Rossin, R.; Schenning, A.; Nicolay, K.; Milroy, L.G.; Robillard, M.S.; Brunsveld, L. Tetrazine-trans-Cyclooctene Chemistry Applied to Fabricate Self-Assembled Fluorescent and Radioactive Nanoparticles for in Vivo Dual Mode Imaging. Bioconjug. Chem. 2019, 30, 547–551. [Google Scholar] [CrossRef]
- Harmsen, S.; Medine, E.I.; Moroz, M.; Nurili, F.; Lobo, J.; Dong, Y.Y.; Turkekul, M.; Pillarsetty, N.V.K.; Ting, R.; Ponomarev, V.; et al. A dual-modal PET/near infrared fluorescent nanotag for long-term immune cell tracking. Biomaterials 2021, 269, 8. [Google Scholar] [CrossRef]
- Rasekholghol, A.; Fazaeli, Y.; Moradi Dehaghi, S.; Ashtari, P. Grafting of CdTe quantum dots on thiol functionalized MCM-41 mesoporous silica for 68Ga radiolabeling: Introducing a novel PET agent. J. Radioanal. Nucl. Chem. 2020, 324, 599–608. [Google Scholar] [CrossRef]
- Shi, S.X.; Chen, F.; Goel, S.Y.; Graves, S.A.; Luo, H.M.; Theuer, C.P.; Engle, J.W.; Cai, W.B. In Vivo Tumor-Targeted Dual-Modality PET/Optical Imaging with a Yolk/Shell-Structured Silica Nanosystem. Nanomicro Lett. 2018, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Licciardello, N.; Hunoldt, S.; Bergmann, R.; Singh, G.; Mamat, C.; Faramus, A.; Ddungu, J.L.Z.; Silvestrini, S.; Maggini, M.; De Cola, L.; et al. Biodistribution studies of ultrasmall silicon nanoparticles and carbon dots in experimental rats and tumor mice. Nanoscale 2018, 10, 9880–9891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.; Ddungu, J.L.Z.; Licciardello, N.; Bergmann, R.; De Cola, L.; Stephan, H. Ultrasmall silicon nanoparticles as a promising platform for multimodal imaging. Faraday Discuss. 2020, 222, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; van der Meulen, N.P.; Wangler, C.; Schibli, R.; Wangler, B. Targeted Cu-64-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Cui, X.J.; Mathe, D.; Kovacs, N.; Horvath, I.; Jauregui-Osoro, M.; de Rosales, R.T.M.; Mullen, G.E.D.; Wong, W.; Yan, Y.; Kruger, D.; et al. Synthesis, Characterization, and Application of Core Shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) Nanoparticle as Trimodal (MRI, PET/SPECT, and Optical) Imaging Agents. Bioconjug. Chem. 2016, 27, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nsubuga, A.; Zarschler, K.; Sgarzi, M.; Graham, B.; Stephan, H.; Joshi, T. Towards Utilising Photocrosslinking of Polydiacetylenes for the Preparation of “Stealth” Upconverting Nanoparticles. Angew. Chem. Int. Ed. 2018, 57, 16036–16040. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, Y.; Zare, H.; Karimi, S.; Feizi, S. 68Ga CdTe/CdS fluorescent quantum dots for detection of tumors: Investigation on the effect of nanoparticle size on stability and in vivo pharmacokinetics. Radiochim. Acta 2020, 108, 565–572. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Thakare, V.; Tran, V.-L.; Natuzzi, M.; Thomas, E.; Moreau, M.; Romieu, A.; Collin, B.; Courteau, A.; Vrigneaud, J.-M.; Louis, C.; et al. Functionalization of theranostic AGuIX® nanoparticles for PET/MRI/optical imaging. RSC Adv. 2019, 9, 24811–24815. [Google Scholar] [CrossRef] [Green Version]
- Cusin, F.; Fernandes Azevedo, L.; Bonnaventure, P.; Desmeules, J.; Daali, Y.; Pastor, C.M. Hepatocyte Concentrations of Indocyanine Green Reflect Transfer Rates Across Membrane Transporters. Basic Clin. Pharmacol. Toxicol. 2017, 120, 171–178. [Google Scholar] [CrossRef]
- Jing, B.; Qian, R.; Jiang, D.; Gai, Y.; Liu, Z.; Guo, F.; Ren, S.; Gao, Y.; Lan, X.; An, R. Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery. J. Nanobiotechnol. 2021, 19, 151. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.K.; Koshy, P.; Yang, J.-L.; Sorrell, C.C. Preclinical Cancer Theranostics—From Nanomaterials to Clinic: The Missing Link. Adv. Funct. Mater. 2021, 31, 2104199. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Shetty, A.; Chandra, S. Inorganic hybrid nanoparticles in cancer theranostics: Understanding their combinations for better clinical translation. Mater. Today Chem. 2020, 18, 100381. [Google Scholar] [CrossRef]
- Nanotechnology Guidance Documents. Available online: https://www.fda.gov/science-research/nanotechnology-programs-fda/nanotechnology-guidance-documents (accessed on 27 March 2022).
- van Leeuwen, F.W.B.; Valdés-Olmos, R.; Buckle, T.; Vidal-Sicart, S. Hybrid surgical guidance based on the integration of radionuclear and optical technologies. Br. J. Radiol. 2016, 89, 20150797. [Google Scholar] [CrossRef]
- Van Oosterom, M.N.; Rietbergen, D.D.D.; Welling, M.M.; Van Der Poel, H.G.; Maurer, T.; Van Leeuwen, F.W.B. Recent advances in nuclear and hybrid detection modalities for image-guided surgery. Expert Rev. Med. Devices 2019, 16, 711–734. [Google Scholar] [CrossRef] [Green Version]
- de Jong, M.; van Leeuwen, F.W.B.; Lahoutte, T.; Evangelista, L.; Barbet, J.; Del Vecchio, S.; Schibli, R. Molecular imaging: The emerging role of optical imaging in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2150–2153. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, O.R.; van den Berg, N.S.; Mathéron, H.M.; van der Poel, H.G.; van Rhijn, B.W.; Bex, A.; van Tinteren, H.; Valdés Olmos, R.A.; van Leeuwen, F.W.B.; Horenblas, S. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur. Urol. 2014, 65, 600–609. [Google Scholar] [CrossRef]
- Christensen, A.; Juhl, K.; Charabi, B.; Mortensen, J.; Kiss, K.; Kjær, A.; von Buchwald, C. Feasibility of Real-Time Near-Infrared Fluorescence Tracer Imaging in Sentinel Node Biopsy for Oral Cavity Cancer Patients. Ann. Sur. Oncol. 2016, 23, 565–572. [Google Scholar] [CrossRef] [Green Version]
- KleinJan, G.H.; van den Berg, N.S.; Brouwer, O.R.; de Jong, J.; Acar, C.; Wit, E.M.; Vegt, E.; van der Noort, V.; Valdés Olmos, R.A.; van Leeuwen, F.W.B.; et al. Optimisation of Fluorescence Guidance During Robot-assisted Laparoscopic Sentinel Node Biopsy for Prostate Cancer. Eur. Urol. 2014, 66, 991–998. [Google Scholar] [CrossRef] [PubMed]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.J.M.A.A.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The best of both worlds: A hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, P.; Vidal-Sicart, S.; Campos, F.; Tapias, A.; Sánchez, N.; Martínez, S.; Carballo, L.; Pahisa, J.; Torné, A.; Ordi, J.; et al. Role of ICG-99mTc-nanocolloid for sentinel lymph node detection in cervical cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Verbeek, F.P.R.; Rietbergen, D.D.D.; Hiel, B.; Vorst, J.R.; Liefers, G.J.; Frangioni, J.V.; Velde, C.J.H.; van Leeuwen, F.W.B.; Vahrmeijer, A.L. Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br. J. Surg. 2013, 100, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Stoffels, I.; Leyh, J.; Pöppel, T.; Schadendorf, D.; Klode, J. Evaluation of a radioactive and fluorescent hybrid tracer for sentinel lymph node biopsy in head and neck malignancies: Prospective randomized clinical trial to compare ICG-99mTc-nanocolloid hybrid tracer versus 99mTc-nanocolloid. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1631–1638. [Google Scholar] [CrossRef]
- Hensbergen, A.W.; van Willigen, D.M.; van Beurden, F.; van Leeuwen, P.J.; Buckle, T.; Schottelius, M.; Maurer, T.; Wester, H.-J.; van Leeuwen, F.W.B. Image-Guided Surgery: Are We Getting the Most Out of Small-Molecule Prostate-Specific-Membrane-Antigen-Targeted Tracers? Bioconjug. Chem. 2020, 31, 375–395. [Google Scholar] [CrossRef]
- Schottelius, M.; Wurzer, A.; Wissmiller, K.; Beck, R.; Koch, M.; Gorpas, D.; Notni, J.; Buckle, T.; van Oosterom, M.N.; Steiger, K.; et al. Synthesis and Preclinical Characterization of the PSMA-Targeted Hybrid Tracer PSMA-I&F for Nuclear and Fluorescence Imaging of Prostate Cancer. J. Nucl. Med. 2019, 60, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Santini, C.; Kuil, J.; Bunschoten, A.; Pool, S.; de Blois, E.; Ridwan, Y.; Essers, J.; Bernsen, M.R.; van Leeuwen, F.W.B.; de Jong, M. Evaluation of a Fluorescent and Radiolabeled Hybrid Somatostatin Analog In Vitro and in Mice Bearing H69 Neuroendocrine Xenografts. J. Nucl. Med. 2016, 57, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Aldrich, M.B.; Marshall, M.V.; Sevick-Muraca, E.M. Preclinical characterization and validation of a dual-labeled trastuzumab-based imaging agent for diagnosing breast cancer. Chin. J. Cancer Res. 2015, 27, 74–82. [Google Scholar] [CrossRef]
- Hekman, M.C.; Rijpkema, M.; Muselaers, C.H.; Oosterwijk, E.; Hulsbergen-Van de Kaa, C.A.; Boerman, O.C.; Oyen, W.J.; Langenhuijsen, J.F.; Mulders, P.F. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics 2018, 8, 2161–2170. [Google Scholar] [CrossRef]
- Ciarrocchi, E.; Belcari, N. Cerenkov luminescence imaging: Physics principles and potential applications in biomedical sciences. EJNMMI Phys. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Thorek, D.L.J.; Grimm, J. Chapter Six—Cerenkov Imaging. In Advances in Cancer Research; Pomper, M.G., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 124, pp. 213–234. [Google Scholar]
- Spinelli, A.E.; Boschi, F. Novel biomedical applications of Cerenkov radiation and radioluminescence imaging. Phys. Med. 2015, 31, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chin, P.T.K.; Welling, M.M.; Meskers, S.C.J.; Valdes Olmos, R.A.; Tanke, H.; van Leeuwen, F.W.B. Optical imaging as an expansion of nuclear medicine: Cerenkov-based luminescence vs fluorescence-based luminescence. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Hustinx, R. Physician centred imaging interpretation is dying out—Why should I be a nuclear medicine physician? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2708–2714. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubeil, M.; Martínez, I.I.S.; Bachmann, M.; Kopka, K.; Tuck, K.L.; Stephan, H. Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 432. https://doi.org/10.3390/ph15040432
Kubeil M, Martínez IIS, Bachmann M, Kopka K, Tuck KL, Stephan H. Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives. Pharmaceuticals. 2022; 15(4):432. https://doi.org/10.3390/ph15040432
Chicago/Turabian StyleKubeil, Manja, Irma Ivette Santana Martínez, Michael Bachmann, Klaus Kopka, Kellie L. Tuck, and Holger Stephan. 2022. "Dual-Labelling Strategies for Nuclear and Fluorescence Molecular Imaging: Current Status and Future Perspectives" Pharmaceuticals 15, no. 4: 432. https://doi.org/10.3390/ph15040432