Daphne jejudoensis Attenuates LPS-Induced Inflammation by Inhibiting TNF-α, IL-1β, IL-6, iNOS, and COX-2 Expression in Periodontal Ligament Cells
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of DJLE on hPDLCs
2.2. Development of an In Vitro Inflammatory Cell Model Using LPS on hPDLCs
2.3. Determination of the Optimal DJLE Concentration for the Experiment on hPDLCs
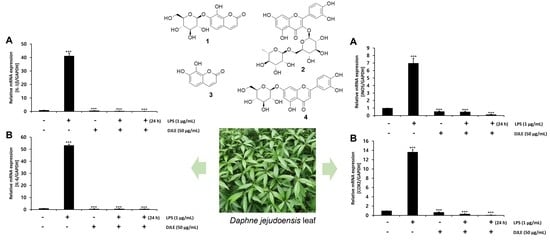
2.4. Effect of DJLE on the Expression of TNF-α in LPS-Induced hPDLCs
2.5. Effect of DJLE on the Expression of IL-1β and IL-6 in LPS-Induced hPDLCs
2.6. Effect of DJLE on the Expression of iNOS and COX-2 in LPS-Induced hPDLCs
2.7. Antimicrobacterial Effect of DJLE on Porphyromonas gingivalis
2.8. Identification of Major Compounds in DJLE
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Sample Preparation
4.4. Isolation and Cell Culture of hPDLCs
4.5. Cytotoxicity of DJLE on hPDLCs
4.6. Anti-Inflammatory Effect of DJLE on hPDLCs
4.7. Reverse Transcription–Polymerase Chain Reaction and Real-Time Polymerase Chain Reaction Analyses
4.8. Antimicrobacterial Effect of DJLE on Porphyromonas gingivalis
4.9. Chemical Profiling of DJLE and Isolation of a Major Compound
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Su, X.; Liu, X.; Ren, K.; Ning, C.; Zhang, Q.; Zhang, S. Daphnes Cortex and its licorice-processed products suppress inflammation via the TLR4/NF-κB/NLRP3 signaling pathway and regulation of the metabolic profile in the treatment of rheumatoid arthritis. J. Ethnopharmacol. 2022, 283, 114657. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassebaum, N.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Brauchle, F.; Noack, M.; Reich, E. Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int. Dental J. 2013, 63, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Time to Take Periodontitis Seriously; Br. Med. J. 2014, 348, g2645. [Google Scholar]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [Green Version]
- Boukortt, K.N.; Saidi-Ouahrani, N.; Boukerzaza, B.; Ouhaibi-Djellouli, H.; Hachmaoui, K.; Benaissa, F.Z.; Taleb, L.; Drabla-Ouahrani, H.; Deba, T.; Ouledhamou, S.A. Association analysis of the IL-1 gene cluster polymorphisms with aggressive and chronic periodontitis in the Algerian population. Arch. Oral Biol. 2015, 60, 1463–1470. [Google Scholar] [CrossRef]
- Ding, C.; Ji, X.; Chen, X.; Xu, Y.; Zhong, L. TNF-α gene promoter polymorphisms contribute to periodontitis susceptibility: Evidence from 46 studies. J. Clin. Periodontol. 2014, 41, 748–759. [Google Scholar] [CrossRef]
- Li, Z.-G.; Li, J.-J.; Sun, C.-A.; Jin, Y.; Wu, W.-W. Interleukin-18 promoter polymorphisms and plasma levels are associated with increased risk of periodontitis: A meta-analysis. Inflamm. Res. 2014, 63, 45–52. [Google Scholar] [CrossRef]
- Tanaka, K.; Miyake, Y.; Hanioka, T.; Furukawa, S.; Miyatake, N.; Arakawa, M. The IL18 promoter polymorphism, rs1946518, is associated with the risk of periodontitis in Japanese women: The Kyushu Okinawa maternal and child health study. Tohoku J. Exp. Med. 2017, 243, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Alayan, J.; Gemmell, E.; Ford, P.; Hamlet, S.; Bird, P.; Ivanovski, S.; Farah, C. The role of cytokines in a Porphyromonas gingivalis-induced murine abscess model. Oral Microbiol. Immunol. 2007, 22, 304–312. [Google Scholar] [CrossRef]
- Eskan, M.A.; Jotwani, R.; Abe, T.; Chmelar, J.; Lim, J.-H.; Liang, S.; Ciero, P.A.; Krauss, J.L.; Li, F.; Rauner, M. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 2012, 13, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Slots, J.; Bragd, L.; Wikström, M.; Dahlén, G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J. Clin. Periodontol. 1986, 13, 570–577. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodontal Res. 1993, 28, 500–510. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Oseko, F.; Nakamura, T.; Imanishi, J.; Kanamura, N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J. Periodontal Res. 2006, 41, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The biological efficacy of natural products against acute and chronic inflammatory diseases in the oral region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.N.; Malaiappan, S. Herbs as an antioxidant arsenal for periodontal diseases. J. Intercult. Ethnopharm. 2016, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Benso, B.; Rosalen, P.L.; Alencar, S.M.; Murata, R.M. Malva sylvestris inhibits inflammatory response in oral human cells. An in vitro infection model. PLoS ONE 2015, 10, e0140331. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.-H.; Hong, S.-P. The taxonomic consideration of leaf epidermal microstructure in Korean Thymelaeaceae Adans. Korean J. Plant Taxon. 2003, 33, 421–433. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.-H.; So, S.; Choi, C.; Kim, M. A new species of Daphne (Thymelaeaceae): D. jejudoensis M. Kim. Korean J. Plant Taxon. 2013, 43, 94–98. [Google Scholar] [CrossRef]
- Mathew, B. 131. DAPHNE KIUSIANA: Thymelaeaceae. Kew Mag. 1989, 6, 112–115. [Google Scholar]
- Hu, X.; Jin, H.; Xu, W.; Zhang, W.; Liu, X.; Yan, S.; Chen, M.; Li, J.; Zhang, W.D. Anti-inflammatory and analgesic effects of Daphne retusa Hemsl. J. Ethnopharmacol. 2008, 120, 118–122. [Google Scholar] [CrossRef]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 104540. [Google Scholar] [CrossRef]
- Park, B.Y.; Min, B.S.; Oh, S.R.; Kim, J.H.; Bae, K.H.; Lee, H.K. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. Phytother. Res. 2006, 20, 610–613. [Google Scholar] [CrossRef]
- Yeşilada, E.; Taninaka, H.; Takaishi, Y.; Honda, G.; Sezik, E.; Momota, H.; Ohmoto, Y.; Taki, T. In vitro inhibitory effects of Daphne oleoides ssp. oleoides on inflammatory cytokines and activity-guided isolation of active constituents. Cytokine 2001, 13, 359–364. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lin, Y.-T.; Chiou, Y.-N.; Wen, K.-C.; Liao, C.-H. Determination of flavonoids in Daphnis Genkwae Flos by high performance liquid chromatography. J. Food Drug Anal. 2001, 9, 1–5. [Google Scholar] [CrossRef]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Ryu, H.W.; Lee, J.W.; Kim, M.O.; Lee, R.W.; Kang, M.J.; Kim, S.M.; Min, J.H.; Oh, E.S.; Song, Y.N.; Jung, S.; et al. Daphnodorin C isolated from the stems of Daphne kiusiana Miquel attenuates airway inflammation in a mouse model of chronic obstructive pulmonary disease. Phytomedicine 2022, 96, 153848. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Park, B.-Y.; Kwon, O.-K.; Yuk, J.-E.; Oh, S.-R.; Kim, H.-S.; Lee, H.-K.; Ahn, K.-S. Anti-inflammatory activity of (−)-aptosimon isolated from Daphne genkwa in RAW264.7 cells. Int. Immunopharmacol. 2009, 9, 878–885. [Google Scholar] [CrossRef]
- Fu, J.-Y.; Masferrer, J.; Seibert, K.; Raz, A.; Needleman, P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 1990, 265, 16737–16740. [Google Scholar] [CrossRef]
- Satô, M.; Hasegawa, M. Conversion of daphnin to daphnetin-8-glucoside in Daphne odora. Phytochemistry 1969, 8, 1211–1214. [Google Scholar] [CrossRef]
- Ueno, K.; Sato, M.; Saito, N. The crystal and molecular structure of daphnin dihydrate: 7-(β-D-glucopyranosyloxy)-8-hydroxycoumarin dihydrate. Bull. Chem. Soc. Jpn. 1983, 56, 1577–1580. [Google Scholar] [CrossRef] [Green Version]
- Cottigli, F.; Loy, G.; Garau, D.; Floris, C.; Caus, M.; Pompei, R.; Bonsignore, L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine 2001, 8, 302–305. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, W.T.; Lim, W.J.; Cho, K.M. Phenolic contents and antioxidant activities from different tissues of Baekseohyang (Daphne kiusiana). Food Sci. Biotechnol. 2011, 20, 695–702. [Google Scholar] [CrossRef]
- Taninaka, H.; Takaishi, Y.; Honda, G.; Imakura, Y.; Sezik, E.; Yesilada, E. Terpenoids and aromatic compounds from Daphne oleoides ssp. oleoides. Phytochemistry 1999, 52, 1525–1529. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Liu, R.; Zhang, C.; Chen, H.; Fu, P.; Shan, L.; Zhang, W. Coumarins from the bark of Daphne marginata. Chem. Nat. Compd. 2007, 43, 317–318. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Matkowski, A.; Hadzik, J.; Dobrowolska-Czopor, B.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis—Antibacterial Effects. Nutrients 2021, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Liu, J.; Mi, Y.; Zhou, D.; Chen, G.; Liang, D.; Li, N.; Hou, Y. Acutissimalignan B from traditional herbal medicine Daphne kiusiana var. atrocaulis (Rehd.) F. Maekawa inhibits neuroinflammation via NF-κB Signaling pathway. Phytomedicine 2021, 84, 153508. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Feng, Y.; Tian, J.-M.; Lu, M.; Xiong, Z.; Zhang, W.-D. Coumarins from Daphne feddei and their potential anti-inflammatory activities. J. Asian Nat. Prod. Res. 2011, 13, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Shan, J.; Di, L.; Jiang, L.; Xu, H. Therapeutic effects of daphnetin on adjuvant-induced arthritic rats. J. Ethnopharmacol. 2008, 120, 259–263. [Google Scholar] [CrossRef]
- Yao, R.; Fu, Y.; Li, S.; Tu, L.; Zeng, X.; Kuang, N. Regulatory effect of daphnetin, a coumarin extracted from Daphne odora, on the balance of Treg and Th17 in collagen-induced arthritis. Eur. J. Pharmacol. 2011, 670, 286–294. [Google Scholar] [CrossRef]
- Choung, H.-W.; Lee, D.-S.; Park, Y.-H.; Lee, Y.S.; Bai, S.; Yoo, S.-H.; Lee, J.-H.; You, H.-K.; Park, J.-C. The effect of CPNE7 on periodontal regeneration. Connect. Tissue Res. 2019, 60, 419–430. [Google Scholar] [CrossRef]










| Gene | Primer (5′-3′) | |
|---|---|---|
| hTNF-α | forward | CCCAGGGACCTCTCTCTAATC |
| reverse | ATGGGCTACAGGCTTGTCACT | |
| hIL-1β | forward | ACGCTCCGGGACTCACAGCA |
| reverse | TGAGGCCCAAGGCCACAGGT | |
| hIL-6 | forward | AGGAGACTTGCCTGGTGAAA |
| reverse | GCATTTGTGGTTGGGTCAGG | |
| hiNOS | forward | GTTCTCAAGGCACAGGTCTC |
| reverse | GCAGGTCACTTATGTCACTTATC | |
| hCOX2 | forward | TTCTCCTTGAAAGGACTTATGGGTAA |
| reverse | AGAACTTGCATTGATGGTGACTGTTT | |
| hGAPDH | forward | CCATGGAGAAGGCTGGGG |
| reverse | CAAAGTTCTCATGGATGACC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-Y.; Lee, D.-S.; Cho, Y.K.; Lee, J.-Y.; Park, J.-H.; Lee, S.H. Daphne jejudoensis Attenuates LPS-Induced Inflammation by Inhibiting TNF-α, IL-1β, IL-6, iNOS, and COX-2 Expression in Periodontal Ligament Cells. Pharmaceuticals 2022, 15, 387. https://doi.org/10.3390/ph15040387
Bae J-Y, Lee D-S, Cho YK, Lee J-Y, Park J-H, Lee SH. Daphne jejudoensis Attenuates LPS-Induced Inflammation by Inhibiting TNF-α, IL-1β, IL-6, iNOS, and COX-2 Expression in Periodontal Ligament Cells. Pharmaceuticals. 2022; 15(4):387. https://doi.org/10.3390/ph15040387
Chicago/Turabian StyleBae, Ji-Yeong, Dong-Seol Lee, You Kyoung Cho, Ji-Yeon Lee, Joo-Hwang Park, and Sang Ho Lee. 2022. "Daphne jejudoensis Attenuates LPS-Induced Inflammation by Inhibiting TNF-α, IL-1β, IL-6, iNOS, and COX-2 Expression in Periodontal Ligament Cells" Pharmaceuticals 15, no. 4: 387. https://doi.org/10.3390/ph15040387