Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance
Abstract
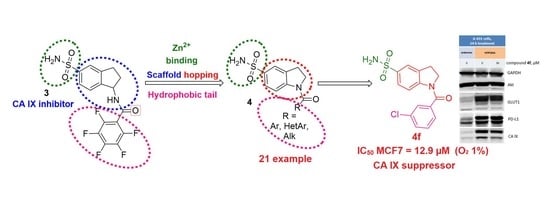
:1. Introduction
2. Results
2.1. Chemistry
2.2. Carbonic Anhydrase Inhibition Assay
2.3. Docking Studies
2.4. Antiproliferative Activity of Indoline-5-sulfonamides
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. Instruments and General Information
4.1.2. 1-Acetylindoline (6)
4.1.3. 1-Acetyl-5-(chlorosulfonyl)indoline (7)
4.1.4. 1-Acetylindoline-5-sulfonamide (8)
4.1.5. Indoline-5-sulfonamide (9)
4.1.6. 1-Benzoylindoline-5-sulfonamide (4a)
4.1.7. 1-(3-Fluorobenzoyl)indoline-5-sulfonamide (4b)
4.1.8. 1-(4-Fluorobenzoyl)indoline-5-sulfonamide (4c)
4.1.9. 1-(2,6-Difluorobenzoyl)indoline-5-sulfonamide (4d)
4.1.10. 1-(Perfluorobenzoyl)indoline-5-sulfonamide (4e)
4.1.11. 1-(3-Chlorobenzoyl)indoline-5-sulfonamide (4f)
4.1.12. 1-(4-Chlorobenzoyl)indoline-5-sulfonamide (4g)
4.1.13. 1-(3,4-Dichlorobenzoyl)indoline-5-sulfonamide (4h)
4.1.14. 1-(3-Methylbenzoyl)indoline-5-sulfonamide (4i)
4.1.15. 1-(3-Methoxybenzoyl)indoline-5-sulfonamide (4j)
4.1.16. 1-(4-Methoxybenzoyl)indoline-5-sulfonamide (4k)
4.1.17. 1-(2,4-Dimethoxybenzoyl)indoline-5-sulfonamide (4l)
4.1.18. 1-(3,4,5-Trimethoxybenzoyl)indoline-5-sulfonamide (4m)
4.1.19. 1-(4-(Methylthio)benzoyl)indoline-5-sulfonamide (4n)
4.1.20. 1-(2-Nitrobenzoyl)indoline-5-sulfonamide (4o)
4.1.21. 1-(4-Nitrobenzoyl)indoline-5-sulfonamide (4p)
4.1.22. 1-Isonicotinoylindoline-5-sulfonamide (4q)
4.1.23. 1-(Thiophene-2-carbonyl)indoline-5-sulfonamide (4r)
4.1.24. 1-(Cyclopentanecarbonyl)indoline-5-sulfonamide (4s)
4.1.25. 1-(2-Cyclopentylacetyl)indoline-5-sulfonamide (4t)
4.1.26. 1-(Cyclohexanecarbonyl)indoline-5-sulfonamide (4u)
4.1.27. 1-Benzylindoline-5-sulfonamide (10)
4.2. CA Inhibitory Assay
4.3. Molecular Modelling Studies
4.4. Cells and Antiproliferative Evaluation
4.5. Statistical Analysis
4.6. Immunoblotting
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Zheng, T.; Song, R.; Wang, J.; Yin, D.; Wang, L.; Liu, H.; Tian, L.; Fang, X.; Meng, X.; et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology 2013, 57, 1847–1857. [Google Scholar] [CrossRef]
- Xu, K.; Zhan, Y.; Yuan, Z.; Qiu, Y.; Wang, H.; Fan, G.; Wang, J.; Li, W.; Cao, Y.; Shen, X.; et al. Hypoxia Induces Drug Resistance in Colorectal Cancer through the HIF-1α/miR-338-5p/IL-6 Feedback Loop. Mol. Ther. 2019, 27, 1810–1824. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Bridges, E.; Valli, A.; Choudhry, H.; Sheldon, H.; Wigfield, S.; Gray, N.; Zois, C.E.; Grimm, F.; Jones, D.; et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 12452–12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Švastová, E.; Hulíková, A.; Rafajová, M.; Zat’Ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Mellor, H.R.; Callaghan, R. Accumulation and distribution of doxorubicin in tumour spheroids: The influence of acidity and expression of P-glycoprotein. Cancer Chemother. Pharmacol. 2011, 68, 1179–1190. [Google Scholar] [CrossRef]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef] [Green Version]
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2008, 69, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Becker, H.M. Carbonic anhydrase IX and acid transport in cancer. Br. J. Cancer 2020, 122, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Beasley, N.J.; Watson, P.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.; Turley, H.; Talks, K.L.; Maxwell, P.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar] [PubMed]
- Pastorekova, S.; Gillies, R.J. The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Pastoreková, S.; Pastorek, J. Cancer-related carbonic anhydrase isozymes and their inhibition. In Carbonic Anhydrase; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Angeli, A.; Carta, F.; Nocentini, A.; Winum, J.-Y.; Zalubovskis, R.; Akdemir, A.; Onnis, V.; Eldehna, W.; Capasso, C.; Simone, G.; et al. Carbonic Anhydrase Inhibitors Targeting Metabolism and Tumor Microenvironment. Metabolites 2020, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Liao, S.-Y.; Ivanova, A.; Danilkovitch-Miagkova, A.; Tarasova, N.; Weirich, G.; Merrill, M.J.; Proescholdt, M.A.; Oldfield, E.H.; Lee, J.; et al. Expression of Hypoxia-Inducible Cell-Surface Transmembrane Carbonic Anhydrases in Human Cancer. Am. J. Pathol. 2001, 158, 905–919. [Google Scholar] [CrossRef] [Green Version]
- Nordfors, K.; Haapasalo, J.; Korja, M.; Niemelä, A.; Laine, J.; Parkkila, A.-K.; Pastorekova, S.; Pastorek, J.; Waheed, A.; Sly, W.S.; et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: An association of CA IX with poor prognosis. BMC Cancer 2010, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Trastour, C.; Benizri, E.; Ettore, F.; Ramaioli, A.; Chamorey, E.; Pouysségur, J.; Berra, E. HIF-1α and CA IX staining in invasive breast carcinomas: Prognosis and treatment outcome. Int. J. Cancer 2007, 120, 1451–1458. [Google Scholar] [CrossRef]
- Driessen, A.; Landuyt, W.; Pastorekova, S.; Moons, J.; Goethals, L.; Haustermans, K.; Nafteux, P.; Penninckx, F.; Geboes, K.; Lerut, T.; et al. Expression of Carbonic Anhydrase IX (CA IX), a Hypoxia-Related Protein, Rather Than Vascular-Endothelial Growth Factor (VEGF), a Pro-Angiogenic Factor, Correlates with an Extremely Poor Prognosis in Esophageal and Gastric Adenocarcinomas. Ann. Surg. 2006, 243, 334–340. [Google Scholar] [CrossRef]
- Loncaster, J.A.; Harris, A.L.; Davidson, S.E.; Logue, J.P.; Hunter, R.D.; Wycoff, C.C.; Pastorek, J.; Ratcliffe, P.J.; Stratford, I.J.; West, C.M. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: Correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001, 61, 333–340. [Google Scholar]
- Wykoff, C.C.; Beasley, N.; Watson, P.H.; Campo, L.; Chia, S.K.; English, R.; Pastorek, J.; Sly, W.S.; Ratcliffe, P.; Harris, A.L. Expression of the Hypoxia-Inducible and Tumor-Associated Carbonic Anhydrases in Ductal Carcinoma in Situ of the Breast. Am. J. Pathol. 2001, 158, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Kivelä, A.; Parkkila, S.; Saarnio, J.; Karttunen, T.J.; Kivelä, J.; Parkkila, A.-K.; Waheed, A.; Sly, W.S.; Grubb, J.H.; Shah, G.; et al. Expression of a Novel Transmembrane Carbonic Anhydrase Isozyme XII in Normal Human Gut and Colorectal Tumors. Am. J. Pathol. 2000, 156, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Watson, P.H.; Chia, S.K.; Wykoff, C.C.; Han, C.; Leek, R.D.; Sly, W.S.; Gatter, K.C.; Ratcliffe, P.; Harris, A.L. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br. J. Cancer 2003, 88, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Ochi, F.; Shiozaki, A.; Ichikawa, D.; Fujiwara, H.; Nakashima, S.; Takemoto, K.; Kosuga, T.; Konishi, H.; Komatsu, S.; Okamoto, K.; et al. Carbonic Anhydrase XII as an Independent Prognostic Factor in Advanced Esophageal Squamous Cell Carcinoma. J. Cancer 2015, 6, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, M.-H.; Ying, T.-H.; Hsieh, Y.-H.; Lin, C.-H.; Shih, C.-H.; Wei, L.-H.; Yang, S.-F. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncol. 2012, 48, 417–423. [Google Scholar] [CrossRef]
- Guerrini, G.; Criscuoli, M.; Filippi, I.; Naldini, A.; Carraro, F. Inhibition of smoothened in breast cancer cells reduces CAXII expression and cell migration. J. Cell. Physiol. 2018, 233, 9799–9811. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-J.; Chen, K.-S.; Chiou, H.-L.; Hsieh, Y.-S. Carbonic anhydrase XII promotes invasion and migration ability of MDA-MB-231 breast cancer cells through the p38 MAPK signaling pathway. Eur. J. Cell Biol. 2010, 89, 598–606. [Google Scholar] [CrossRef]
- Kopecka, J.; Campia, I.; Jacobs, A.; Frei, A.P.; Ghigo, D.; Wollscheid, B.; Riganti, C. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 2015, 6, 6776–6793. [Google Scholar] [CrossRef] [Green Version]
- Salaroglio, I.C.; Mujumdar, P.; Annovazzi, L.; Kopecka, J.; Mellai, M.; Schiffer, D.; Poulsen, S.-A.; Riganti, C. Carbonic Anhydrase XII Inhibitors Overcome P-Glycoprotein–Mediated Resistance to Temozolomide in Glioblastoma. Mol. Cancer Ther. 2018, 17, 2598–2609. [Google Scholar] [CrossRef] [Green Version]
- Mujumdar, P.; Kopecka, J.; Bua, S.; Supuran, C.T.; Riganti, C.; Poulsen, S.-A. Carbonic Anhydrase XII Inhibitors Overcome Temozolomide Resistance in Glioblastoma. J. Med. Chem. 2019, 62, 4174–4192. [Google Scholar] [CrossRef] [Green Version]
- Nocentini, A.; Angeli, A.; Carta, F.; Winum, J.-Y.; Zalubovskis, R.; Carradori, S.; Capasso, C.; Donald, W.A.; Supuran, C.T. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J. Enzym. Inhib. Med. Chem. 2021, 36, 561–580. [Google Scholar] [CrossRef]
- Berrino, E.; Michelet, B.; Martin-Mingot, A.; Carta, F.; Supuran, C.T.; Thibaudeau, S. Modulating the Efficacy of Carbonic Anhydrase Inhibitors through Fluorine Substitution. Angew. Chem. 2021, 60, 23068–23082. [Google Scholar] [CrossRef]
- Supuran, C.T. Emerging role of carbonic anhydrase inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.C.; Chafe, S.C.; Supuran, C.T.; Dedhar, S. Cancer Therapeutic Targeting of Hypoxia Induced Carbonic Anhydrase IX: From Bench to Bedside. Cancers 2022, 14, 3297. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Siwach, K.; Supuran, C.T.; Sharma, P.K. A decade of tail-approach based design of selective as well as potent tumor associated carbonic anhydrase inhibitors. Bioorganic Chem. 2022, 126, 105920. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.O.; Petreni, A.; Supuran, C.T.; El-Hamamsy, M.H. Discovery of new carbonic anhydrase IX inhibitors as anticancer agents by toning the hydrophobic and hydrophilic rims of the active site to encounter the dual-tail approach. Eur. J. Med. Chem. 2022, 232, 114190. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.O.; Belal, A.; Abourehab, M.A.S.; Angeli, A.; Bonardi, A.; Supuran, C.T.; El-Hamamsy, M.H. Dependence on linkers’ flexibility designed for benzenesulfonamides targeting discovery of novel hCA IX inhibitors as potent anticancer agents. J. Enzyme Inhib. Med. Chem. 2022, 37, 2765–2785. [Google Scholar] [CrossRef]
- Arrighi, G.; Puerta, A.; Petrini, A.; Hicke, F.J.; Nocentini, A.; Fernandes, M.X.; Padrón, J.M.; Supuran, C.T.; Fernández-Bolaños, J.G.; López, Ó. Squaramide-Tethered Sulfonamides and Coumarins: Synthesis, Inhibition of Tumor-Associated CAs IX and XII and Docking Simulations. Int. J. Mol. Sci. 2022, 23, 7685. [Google Scholar] [CrossRef]
- Manzoor, S.; Angeli, A.; Zara, S.; Carradori, S.; Rahman, A.; Raza, K.; Supuran, C.T.; Hoda, N. Development of benzene and benzothiazole-sulfonamide analogues as selective inhibitors of the tumor-associated carbonic anhydrase IX. Eur. J. Med. Chem. 2022, 243, 114793. [Google Scholar] [CrossRef]
- Lock, F.E.; McDonald, P.C.; Lou, Y.; Serrano, I.; Chafe, S.C.; Ostlund, C.; Aparicio, S.; Winum, J.-Y.; Supuran, C.T.; Dedhar, S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013, 32, 5210–5219. [Google Scholar] [CrossRef]
- Pacchiano, F.; Carta, F.; McDonald, P.C.; Lou, Y.; Vullo, D.; Scozzafava, A.; Dedhar, S.; Supuran, C.T. Ureido-Substituted Benzenesulfonamides Potently Inhibit Carbonic Anhydrase IX and Show Antimetastatic Activity in a Model of Breast Cancer Metastasis. J. Med. Chem. 2011, 54, 1896–1902. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Keller, U.A.D.; Leung, S.; Huntsman, D.; et al. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res 2011, 71, 3364–3376. [Google Scholar] [CrossRef] [Green Version]
- Kalinin, S.; Malkova, A.; Sharonova, T.; Sharoyko, V.; Bunev, A.; Supuran, C.T.; Krasavin, M. Carbonic Anhydrase IX Inhibitors as Candidates for Combination Therapy of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 13405. [Google Scholar] [CrossRef]
- Boyd, N.H.; Walker, K.; Fried, J.; Hackney, J.R.; McDonald, P.C.; Benavides, G.A.; Spina, R.; Audia, A.; Scott, S.E.; Libby, C.J.; et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2017, 2, e92928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedlund, E.-M.E.; McDonald, P.C.; Nemirovsky, O.; Awrey, S.; Jensen, L.D.; Dedhar, S. Harnessing Induced Essentiality: Targeting Carbonic Anhydrase IX and Angiogenesis Reduces Lung Metastasis of Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.C.; Chafe, S.C.; Brown, W.S.; Saberi, S.; Swayampakula, M.; Venkateswaran, G.; Nemirovsky, O.; Gillespie, J.A.; Karasinska, J.M.; Kalloger, S.E.; et al. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia. Gastroenterology 2019, 157, 823–837. [Google Scholar] [CrossRef] [Green Version]
- Güzel-Akdemir, Ö.; Demir-Yazıcı, K.; Vullo, D.; Supuran, C.T.; Akdemir, A. New Pyridinium Salt Derivatives of 2-(Hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide as Selective Inhibitors of Tumour-Related Human Carbonic Anhydrase Isoforms IX and XII. Anti-Cancer Agents Med. Chem. 2022, 22, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Goud, N.S.; Swain, B.; Sahoo, S.K.; Choli, A.; Angeli, A.; Kushwah, B.S.; Yaddanapudi, V.M.; Supuran, C.T.; Arifuddin, M. Synthesis of a new series of quinoline/pyridine indole-3-sulfonamide hybrids as selective carbonic anhydrase IX inhibitors. Bioorganic Med. Chem. Lett. 2022, 70, 128809. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2020, 209, 112923. [Google Scholar] [CrossRef] [PubMed]
- Krymov, S.K.; Scherbakov, A.M.; Salnikova, D.I.; Sorokin, D.V.; Dezhenkova, L.G.; Ivanov, I.V.; Vullo, D.; De Luca, V.; Capasso, C.; Supuran, C.T.; et al. Synthesis, biological evaluation, and in silico studies of potential activators of apoptosis and carbonic anhydrase inhibitors on isatin-5-sulfonamide scaffold. Eur. J. Med. Chem. 2021, 228, 113997. [Google Scholar] [CrossRef]
- Thiry, A.; Ledecq, M.; Cecchi, A.; Dogné, J.-M.; Wouters, J.; Supuran, C.T.; Masereel, B. Indanesulfonamides as Carbonic Anhydrase Inhibitors. Toward Structure-Based Design of Selective Inhibitors of the Tumor-Associated Isozyme CA IX. J. Med. Chem. 2006, 49, 2743–2749. [Google Scholar] [CrossRef]
- Dubois, L.; Peeters, S.; Lieuwes, N.G.; Geusens, N.; Thiry, A.; Wigfield, S.; Carta, F.; Mcintyre, A.; Scozzafava, A.; Dogné, J.-M.; et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother. Oncol. 2011, 99, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Langdon, S.R.; Ertl, P.; Brown, N. Bioisosteric Replacement and Scaffold Hopping in Lead Generation and Optimization. Mol. Inform. 2010, 29, 366–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Stumpfe, D.; Bajorath, J. Recent Advances in Scaffold Hopping. J. Med. Chem. 2016, 60, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.-J.; Flohr, A.; Stahl, M. Scaffold hopping. Drug Discov. Today: Technol. 2004, 1, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Cocklin, S. Bioisosteric Replacement as a Tool in Anti-HIV Drug Design. Pharmaceuticals 2020, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Alhadrami, H.A.; Sayed, A.M.; Al-Khatabi, H.; Alhakamy, N.A.; Rateb, M.E. Scaffold Hopping of α-Rubromycin Enables Direct Access to FDA-Approved Cromoglicic Acid as a SARS-CoV-2 MPro Inhibitor. Pharmaceuticals 2021, 14, 541. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Liu, X.; Meng, L.-W.; Li, M.-M.; Li, Y.-R.; Yu, G.-X.; Song, J.; Zhang, H.-Y.; Chen, P.; Zhang, S.-Y.; et al. Discovery of indoline derivatives as anticancer agents via inhibition of tubulin polymerization. Bioorganic Med. Chem. Lett. 2021, 43, 128095. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, A.; Kaur, N.; Ojha, R.; Nepali, K. Steering the antitumor drug discovery campaign towards structurally diverse indolines. Bioorganic Chem. 2019, 94, 103436. [Google Scholar] [CrossRef]
- Fu, D.-J.; Li, M.; Zhang, S.-Y.; Li, J.-F.; Sha, B.; Wang, L.; Zhang, Y.-B.; Chen, P.; Hu, T. Discovery of indoline derivatives that inhibit esophageal squamous cell carcinoma growth by Noxa mediated apoptosis. Bioorganic Chem. 2019, 92, 103190. [Google Scholar] [CrossRef]
- Preobrazhenskaya, M.N. Synthesis of Substituted Indoles via Indolines. Russ. Chem. Rev. 1967, 36, 753–771. [Google Scholar] [CrossRef]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Mishra, C.B.; Tiwari, M.; Supuran, C.T. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: Where are we today? Med. Res. Rev. 2020, 40, 2485–2565. [Google Scholar] [CrossRef]
- Supuran, C.T. Novel carbonic anhydrase inhibitors. Future Med. Chem. 2021, 13, 1935–1937. [Google Scholar] [CrossRef] [PubMed]
- Glamkowski, E.J.; Reitano, P.A. Synthesis and evaluation for diuretic activity of 1-substituted 6-chloro-5-sulfamylindolines. J. Med. Chem. 1979, 22, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Hao, P.; Dutta, B.; Cheow, E.S.H.; Sim, K.H.; Gan, C.S.; Lim, S.K.; Sze, S.K. Hypoxia Modulates A431 Cellular Pathways Association to Tumor Radioresistance and Enhanced Migration Revealed by Comprehensive Proteomic and Functional Studies. Mol. Cell. Proteom. 2013, 12, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Hao, P.; Law, S.K.A.; Sze, S.K. Hypoxia-induced Changes to Integrin α 3 Glycosylation Facilitate Invasion in Epidermoid Carcinoma Cell Line A431. Mol. Cell. Proteom. 2014, 13, 3126–3137. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Pandey, C.; Sze, S.K.; Thanabalu, T. Hypoxia Activated EGFR Signaling Induces Epithelial to Mesenchymal Transition (EMT). PLoS ONE 2012, 7, e49766. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.; Thoms, J.; Sykes, J.; Ahmed, O.; Evans, A.; van Rhijn, B.G.; Mirtti, T.; Stakhovskyi, O.; Laato, M.; Margel, D.; et al. Hypoxia Marker GLUT-1 (Glucose Transporter 1) is an Independent Prognostic Factor for Survival in Bladder Cancer Patients Treated with Radical Cystectomy. Bladder Cancer 2016, 2, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.; Mukherjee, D.; Lunj, S.; Choudhury, A.; Hoskin, P.; West, C.; Illidge, T. The effect of hypoxia on PD-L1 expression in bladder cancer. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Teodori, E.; Braconi, L.; Bua, S.; Lapucci, A.; Bartolucci, G.; Manetti, D.; Romanelli, M.N.; Dei, S.; Supuran, C.T.; Coronnello, M. Dual P-Glycoprotein and CA XII Inhibitors: A New Strategy to Reverse the P-gp Mediated Multidrug Resistance (MDR) in Cancer Cells. Molecules 2020, 25, 1748. [Google Scholar] [CrossRef] [Green Version]
- Kopecka, J.; Rankin, G.M.; Salaroglio, I.C.; Poulsen, S.-A.; Riganti, C. P-glycoprotein-mediated chemoresistance is reversed by carbonic anhydrase XII inhibitors. Oncotarget 2016, 7, 85861–85875. [Google Scholar] [CrossRef] [Green Version]
- Borror, A.L.; Chinoporos, E.; Filosa, M.P.; Herchen, S.R.; Petersen, C.P.; Stern, C.A.; Onan, K.D. Regioselectivity of electrophilic aromatic substitution: Syntheses of 6- and 7-sulfamoylindolines and -indoles. J. Org. Chem. 1988, 53, 2047–2052. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of the complete domain of the η-carbonic anhydrase from Plasmodium falciparum. Bioorganic Med. Chem. 2016, 24, 4410–4414. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, S.; Vullo, D.; De Luca, V.; Carginale, V.; di Fonzo, P.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Anion inhibition profiles of α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae. Bioorganic Med. Chem. 2016, 24, 3413–3417. [Google Scholar] [CrossRef]
- De Luca, V.; Vullo, D.; Del Prete, S.; Carginale, V.; Osman, S.M.; AlOthman, Z.; Supuran, C.T.; Capasso, C. Cloning, characterization and anion inhibition studies of a γ-carbonic anhydrase from the Antarctic bacterium Colwellia psychrerythraea. Bioorganic Med. Chem. 2016, 24, 835–840. [Google Scholar] [CrossRef]
- Sagnou, M.; Novikov, F.N.; Ivanova, E.S.; Alexiou, P.; Stroylov, V.S.; Titov, I.Y.; Tatarskiy, V.V.; Vagida, M.S.; Pelecanou, M.; Shtil, A.A.; et al. Novel curcumin derivatives as P-glycoprotein inhibitors: Molecular modeling, synthesis and sensitization of multidrug resistant cells to doxorubicin. Eur. J. Med. Chem. 2020, 198, 112331. [Google Scholar] [CrossRef]
- Shchekotikhin, A.E.; Dezhenkova, L.G.; Susova, O.Y.; Glazunova, V.A.; Luzikov, Y.N.; Sinkevich, Y.B.; Buyanov, V.N.; Shtil, A.A.; Preobrazhenskaya, M.N. Naphthoindole-based analogues of tryptophan and tryptamine: Synthesis and cytotoxic properties. Bioorganic Med. Chem. 2007, 15, 2651–2659. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Lobanova, Y.S.; Shatskaya, V.A.; Onopchenko, O.V.; Gershtein, E.S.; Krasil’nikov, M.A. Activation of mitogenic pathways and sensitization to estrogen-induced apoptosis: Two independent characteristics of tamoxifen-resistant breast cancer cells? Breast Cancer Res. Treat. 2006, 100, 1–11. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 2011, 1, 121–122. [Google Scholar] [CrossRef]









| KI (nM) * | ||||
|---|---|---|---|---|
| Cmp | hCA I | hCA II | hCA IX | hCA XII |
| 4a | 79.0 | 5.4 | >104 | 258.5 |
| 4b | 65.9 | 5.6 | >104 | >104 |
| 4c | 88.3 | 9.5 | 2246.9 | >104 |
| 4d | 268.1 | 9.1 | 1330.2 | 41.3 |
| 4e | 52.2 | 3.4 | 1297.5 | 126.7 |
| 4f | 303.9 | 31.3 | 141.2 | 111.7 |
| 4g | 242.1 | 66.2 | >104 | 57.0 |
| 4h | 350.1 | 54.0 | >104 | 56.0 |
| 4i | 220.1 | 36.1 | >104 | 93.9 |
| 4j | 67.7 | 9.2 | >104 | 110.0 |
| 4k | 77.7 | 9.3 | 173.0 | >104 |
| 4l | 71.5 | 4.0 | 203.2 | 112.5 |
| 4m | 49.0 | 3.0 | >104 | 103.0 |
| 4n | 71.4 | 9.3 | 1990.1 | 91.0 |
| 4o | 83.4 | 4.5 | >104 | 119.6 |
| 4p | 60.5 | 42.2 | 2109.4 | 198.1 |
| 4q | 64.0 | 12.5 | 2394.3 | >104 |
| 4r | 42.2 | 5.0 | 176.7 | 147.3 |
| 4s | 60.9 | 7.1 | 132.8 | 88.1 |
| 4t | 51.6 | 3.5 | 176.8 | >104 |
| 4u | 67.0 | 1.8 | 222.6 | >104 |
| 10 | 41.0 | 3.0 | >104 | >104 |
| AAZ | 250 | 12.1 | 25.7 | 5.7 |
| 3 ** | 770 | 490 | 3.5 | N.T. |
| Compound | IC50 a against MCF7 Cells, µM | |
|---|---|---|
| Normoxia | Hypoxia | |
| 4a | >50 | 36.9 ± 3.5 |
| 4b | >50 | >50 |
| 4c | 40.6 ± 3.8 | 32.7 ± 2.8 |
| 4d | >50 | 35.9 ± 3.4 |
| 4e | 29.9 ± 2.7 | 20.2 ± 2.0 |
| 4f | 24.5 ± 1.9 | 12.9 ± 1.2 |
| 4g | 36.5 ± 3.3 | 24.2 ± 2.1 |
| 4h | 35.2 ± 3.5 | 48.7 ± 4.5 |
| 4i | >50 | 43.6 ± 4.0 |
| 4j | 46.2 ± 3.6 | >50 |
| 4k | >50 | >50 |
| 4l | 37.0 ± 3.0 | >50 |
| 4m | 29.6 ± 2.2 | 36.7 ± 3.2 |
| 4n | 18.4 ± 1.6 | >50 |
| 4o | 43.7 ± 3.7 | 39.5 ± 3.4 |
| 4p | 48.8 ± 4.4 | 39.9 ± 3.5 |
| 4q | >50 | >50 |
| 4r | 47.7 ± 3.8 | 39.1 ± 3.9 |
| 4s | >50 | >50 |
| 4t | 38.0 ± 3.5 | 36.0 ± 3.3 |
| 4u | 37.1 ± 3.5 | 39.2 ± 3.4 |
| 10 | 23.6 ± 2.0 | 34.8 ± 3.2 |
| 3 | >50 | >50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krymov, S.K.; Scherbakov, A.M.; Dezhenkova, L.G.; Salnikova, D.I.; Solov’eva, S.E.; Sorokin, D.V.; Vullo, D.; De Luca, V.; Capasso, C.; Supuran, C.T.; et al. Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance. Pharmaceuticals 2022, 15, 1453. https://doi.org/10.3390/ph15121453
Krymov SK, Scherbakov AM, Dezhenkova LG, Salnikova DI, Solov’eva SE, Sorokin DV, Vullo D, De Luca V, Capasso C, Supuran CT, et al. Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance. Pharmaceuticals. 2022; 15(12):1453. https://doi.org/10.3390/ph15121453
Chicago/Turabian StyleKrymov, Stepan K., Alexander M. Scherbakov, Lyubov G. Dezhenkova, Diana I. Salnikova, Svetlana E. Solov’eva, Danila V. Sorokin, Daniela Vullo, Viviana De Luca, Clemente Capasso, Claudiu T. Supuran, and et al. 2022. "Indoline-5-Sulfonamides: A Role of the Core in Inhibition of Cancer-Related Carbonic Anhydrases, Antiproliferative Activity and Circumventing of Multidrug Resistance" Pharmaceuticals 15, no. 12: 1453. https://doi.org/10.3390/ph15121453