1,6-Naphthyridin-2(1H)-ones: Synthesis and Biomedical Applications
Abstract
:1. Introduction
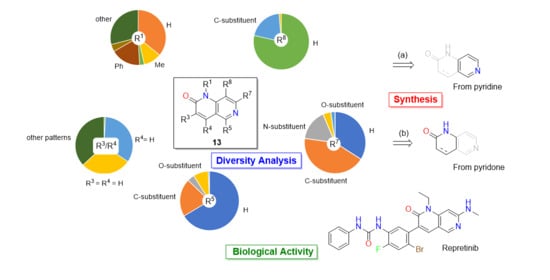
2. Structural Features of 1,6-Naphthyridin-2(1H)-ones: Substitution Patterns and Degree of Unsaturation C3-C4
2.1. Substitution Pattern at N1
2.2. Substitution Pattern at C3 and C4
2.3. Substitution Pattern at C5 and C7
2.4. Substitution Pattern at C8
3. Synthetic Approaches to 1,6-Naphthyridin-2(1H)-ones
- (1)
- Reaction Structure tool: it can be used in two ways, (a) drawing the substructures of two starting products and the reaction arrow without including the reaction product or (b) drawing the substructure of a possible starting material and the reaction arrow together with the structure of the reaction product. Both approaches are useful when the possible starting products are known.
- (2)
- Retrosynthetic analysis tool: drawing the structure of the final product and indicating with a small arrow of the structure editor the bonds to be retrosynthetically broken. This approach is useful for exploring several possible disconnections from a given final structure.
3.1. Synthesis from a Preformed Pyridine
3.2. Synthesis from a Preformed Pyridone
4. Biomedical Applications of 1,6-Naphthyridin-2(1H)-ones
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef]
- Altomare, C.D. Privileged heterocyclic scaffolds in chemical biology and drug discovery: Synthesis and bioactivity. Chem. Heterocycl. Compd. 2017, 53, 239. [Google Scholar] [CrossRef] [Green Version]
- Jubete, G.; De La Bellacasa, R.P.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. Pyrido[2,3-d]pyrimidin-7(8H)-ones: Synthesis and biomedical applications. Molecules 2019, 24, 4161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig de la Bellacasa, R.; Roue, G.; Balsas, P.; Perez-Galan, P.; Teixido, J.; Colomer, D.; Borrell, J.I. 4-Amino-2-arylamino-6-(2,6-dichlorophenyl)-pyrido[2,3-d]pyrimidin-7-(8H)-ones as BCR kinase inhibitors for B lymphoid malignancies. Eur. J. Med. Chem. 2014, 86, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.A.M.; Roman, S.G.; Bilbao, J.I.B.; Closa, J.T.; Tejedor, R.E.; de la Bellacasa, R.P. Use of 4-Amino-6-(2,6-Dichlorophenyl)-8-Methyl-2-(Phenylamino)-Pyrido[2,3-d]Pyrimidin-7(8H)-One in Formulations for Treatment of Solid Tumors. EP Patent 3120851A1, 21 July 2017. [Google Scholar]
- Camarasa, M.; De La Bellacasa, R.P.; González, À.L.; Ondoño, R.; Estrada, R.; Franco, S.; Badia, R.; Esté, J.; Martínez, M.Á.; Teixidó, J.; et al. Design, synthesis and biological evaluation of pyrido[2,3-d]pyrimidin-7-(8H)-ones as HCV inhibitors. Eur. J. Med. Chem. 2016, 115, 463–483. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the Chemistry of Naphthyridines; Elsevier Masson SAS: N.D. Zelinsky Institute of Organic Chemistry, Academy of Sciences: Moscow, Russia, 2006; Volume 91, pp. 189–300. [Google Scholar] [CrossRef]
- Victory, P.J.; Teixido, J.; Borrell, J.I. A synthesis of 1,2,3,4-tetrahydro-1,6-naphthyridines. Heterocycles 1992, 34, 1905–1916. [Google Scholar] [CrossRef]
- Victory, P.J.; Teixido, J.; Borrell, J.I.; Busquets, N. 1,2,3,4-Tetrahydro-1,6-naphthyridines. Part 2. Formation and unexpected reactions of 1,2,3,4-tetrahydro-7H-pyrano[4,3-b]pyridine-2,7-diones. Heterocycles 1993, 36, 1–6. [Google Scholar] [CrossRef]
- Chemical Abstracts Service. Scifinder, Version 2019; Chemical Abstracts Service: Columbus, OH, USA, 2019. [Google Scholar]
- Phuan, P.-W.; Kozlowski, M.C. Product class 8: Naphthyridines. Sci. Synth. 2005, 15, 947–985. [Google Scholar] [CrossRef]
- Sams, A.G.; Larsen, K.; Mikkelsen, G.K.; Hentzer, M.; Christoffersen, C.T.; Jensen, K.G.; Frederiksen, K.; Bang-Andersen, B. Hit-to-lead investigation of a series of novel combined dopamine D2 and muscarinic M1 receptor ligands with putative antipsychotic and pro-cognitive potential. Bioorg. Med. Chem. Lett. 2012, 22, 5134–5140. [Google Scholar] [CrossRef] [PubMed]
- Haglid, F. Syntheses of tetrahydronaphthyridines. Ark. Kemi 1967, 26, 489. [Google Scholar]
- Dong, Z.; Wang, Z.; Guo, Z.-Q.; Gong, S.; Zhang, T.; Liu, J.; Luo, C.; Jiang, H.; Yang, C.-G. Structure—Activity Relationship of SPOP Inhibitors against Kidney Cancer. J. Med. Chem. 2020, 63, 4849–4866. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Sarakha, M.; Mailhot, G. Enhancement of the photocatalytic activity of decatungstate, W10O324-, for the oxidation of sulfasalazine/sulfapyridine in the presence of hydrogen peroxide. J. Photochem. Photobiol. A Chem. 2021, 404, 112890. [Google Scholar] [CrossRef]
- Bowden, B.F.; McCool, B.J.; Willis, R.H. Lihouidine, a novel spiro polycyclic aromatic alkaloid from the marine sponge Suberean. sp. (Aplysinellidae, Verongida). J. Org. Chem. 2004, 69, 7791–7793. [Google Scholar] [CrossRef]
- Huang, X.; Shaffer, P.L.; Ayube, S.; Bregman, H.; Chen, H.; Lehto, S.G.; Luther, J.A.; Matson, D.J.; McDonough, S.I.; Michelsen, K.; et al. Crystal structures of human glycine receptor α3 bound to a novel class of analgesic potentiators. Nat. Struct. Mol. Biol. 2017, 24, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Montoir, D.; Tonnerre, A.; Duflos, M.; Bazin, M.-A. Efficient one-pot synthesis of 3,7-disubstituted 1,6-naphthyridin-2(1H)-ones through regioselective palladium-catalyzed cross-coupling and SNAr reactions. Tetrahedron 2015, 71, 3303–3313. [Google Scholar] [CrossRef]
- Montoir, D.; Barille-Nion, S.; Tonnerre, A.; Juin, P.; Duflos, M.; Bazin, M.-A. Novel 1,6-naphthyridin-2(1H)-ones as potential anticancer agents targeting Hsp90. Eur. J. Med. Chem. 2016, 119, 17–33. [Google Scholar] [CrossRef]
- Vicente-Garcia, E.; Ramon, R.; Preciado, S.; Lavilla, R. Multicomponent reaction access to complex quinolines via oxidation of the Povarov adducts. Beilstein J. Org. Chem. 2011, 7, 980–987. [Google Scholar] [CrossRef]
- Gao, R.-X.; Luan, X.-Q.; Xie, Z.-Y.; Yang, L.; Pei, Y. Fe-Catalyzed decarbonylative cascade reaction of N-aryl cinnamamides with aliphatic aldehydes to construct 3,4-dihydroquinolin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5262–5268. [Google Scholar] [CrossRef]
- Sharma, L.; Tsai, Y.-C.; Liu, A.A.; Liu, L.F.; LaVoie, E.J. Cytotoxicity and TOP1-targeting activity of 8- and 9-amino derivatives of 5-butyl- and 5-(2-N,N-dimethylamino)ethyl-5H-dibenzo[c,h][1,6]naphthyridin-6-ones. Eur. J. Med. Chem. 2009, 44, 1471–1476. [Google Scholar] [CrossRef]
- Manikandan, T.S.; Ramesh, R.; Semeril, D. The Tandem C-H/N-H Activation of N-Methyl Arylamide Catalyzed by Dinuclear Pd(II) Benzhydrazone Complex: A Concise Access to Phenanthridinone. Organometallics 2019, 38, 319–328. [Google Scholar] [CrossRef]
- Chung, J.Y.L.; Cvetovich, R.J.; McLaughlin, M.; Amato, J.; Tsay, F.R.; Jensen, M.; Weissman, S.; Zewge, D. Synthesis of a naphthyridone p38 MAP kinase inhibitor. J. Org. Chem. 2006, 71, 8602–8609. [Google Scholar] [CrossRef]
- Hunt, J.A.; Kallashi, F.; Ruzek, R.D.; Sinclair, P.J.; Ita, I.; McCormick, S.X.; Pivnichny, J.V.; Hop, C.E.C.A.; Kumar, S.; Wang, Z.; et al. p38 Inhibitors: Piperidine- and 4-aminopiperidine-substituted naphthyridinones, quinolinones, and dihydroquinazolinones. Bioorg. Med. Chem. Lett. 2003, 13, 467–470. [Google Scholar] [CrossRef]
- Xu, Z.; Cai, J.; Liu, Y.; Song, B.; Shi, S.; Yao, T.; Hu, L.; Ding, C.Z. Salt Form and Crystal Form of Pyridopyridone Derivative. WO2019238088 A1, 19 December 2019. [Google Scholar]
- Yanagisawa, H.; Shimoji, Y.; Hashimoto, T. Preparation of Indolonaphthyridine Derivatives as Cardiovascular Agents. JP05310738 A, 22 November 1993. [Google Scholar]
- Seixas, J.D.; Sousa, B.B.; Marques, M.C.; Guerreiro, A.; Traquete, R.; Rodrigues, T.; Albuquerque, I.S.; Sousa, M.; Lemos, A.R.; Sousa, P.M.F.; et al. Rationally designed potent BMX inhibitors reveals mode of covalent binding at the atomic level. ChemRxiv 2020, 1–35. [Google Scholar] [CrossRef]
- Li, H.; Sun, W.; Huang, X.; Lu, X.; Patel, P.R.; Kim, M.; Orr, M.J.; Fisher, R.M.; Tanaka, T.Q.; McKew, J.C.; et al. Efficient Synthesis of 1,9-Substituted Benzo[h][1,6]naphthyridin-2(1H)-ones and Evaluation of their Plasmodium falciparum Gametocytocidal Activities. ACS Comb. Sci. 2017, 19, 748–754. [Google Scholar] [CrossRef]
- Shao, C.; Wang, C.; Mou, X.; Xu, R. Preparation of Lactam Compounds Useful as Antiviral Agents. U.S. Patent 20200030312 A1, 30 January 2020. [Google Scholar]
- Shao, C.; Wang, C.; Mou, X.; Xu, R. Lactam Compounds as Antiviral Agents and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Viral Infections. WO Patent 2018177296 A1, 4 October 2018. [Google Scholar]
- Wu, H.; Wang, W.; Liu, F.; Weisberg, E.E.; Tian, B.; Chen, Y.; Li, B.; Wang, A.; Wang, B.; Zhao, Z.; et al. Discovery of a Potent, Covalent BTK Inhibitor for B-Cell Lymphoma. ACS Chem. Biol. 2014, 9, 1086–1091. [Google Scholar] [CrossRef]
- Wang, B.; Deng, Y.; Chen, Y.; Yu, K.; Wang, A.; Liang, Q.; Wang, W.; Chen, C.; Wu, H.; Hu, C.; et al. Structure-activity relationship investigation for benzonaphthyridinone derivatives as novel potent Bruton’s tyrosine kinase (BTK) irreversible inhibitors. Eur. J. Med. Chem. 2017, 137, 545–557. [Google Scholar] [CrossRef]
- Teixido, J.; Borrell, J.I.; Serra, B.; Matallana, J.L.; Colominas, C.; Carrion, F.; Pascual, R.; Falco, J.L.; Batllori, X. Cyclization of tautomeric 1,5-dinitrile systems with hydrogen halides: A definitive mechanistic rationalization? Heterocycles 1999, 50, 739–756. [Google Scholar] [CrossRef]
- Martinez-Teipel, B.; Teixido, J.; Pascual, R.; Mora, M.; Pujola, J.; Fujimoto, T.; Borrell, J.I.; Michelotti, E.L. 2-Methoxy-6-oxo-1,4,5,6-tetrahydropyridine-3-carbonitriles: Versatile Starting Materials for the Synthesis of Libraries with Diverse Heterocyclic Scaffolds. J. Comb. Chem. 2005, 7, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Bregman, H.; Simard, J.R.; Andrews, K.L.; Ayube, S.; Chen, H.; Gunaydin, H.; Guzman-Perez, A.; Hu, J.; Huang, L.; Huang, X.; et al. The Discovery and Hit-to-Lead Optimization of Tricyclic Sulfonamides as Potent and Efficacious Potentiators of Glycine Receptors. J. Med. Chem. 2017, 60, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.K.; Soliman, A.Y.; Abdel-Motaleb, R.M.; Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Fouda, M.M.G.; Al-Deyab, S.S.; Hrdina, R. Synthesis and antibacterial activity of new quinoline derivatives starting from coumarin compounds. J. Pure Appl. Microbiol. 2013, 7, 453–458. [Google Scholar]
- Vicente-Garcia, E.; Catti, F.; Ramon, R.; Lavilla, R. Unsaturated Lactams: New Inputs for Povarov-Type Multicomponent Reactions. Org. Lett. 2010, 12, 860–863. [Google Scholar] [CrossRef]
- Ratcliffe, A.H.; Pearce, R.J.; Gibson, K.H.; Wood, R.; Masek, B.B. Preparation of Naphthyridine Derivatives as Angiotensin II Inhibitors. EP Patent 516392 A2, 2 February 1992. [Google Scholar]
- Sakamoto, T.; Miura, N.; Kondo, Y.; Yamanaka, H. Condensed heteroaromatic ring systems. V. Formal synthesis of matrine and related compounds using palladium-catalyzed carbon-carbon bond formations as key reactions. Chem. Pharm. Bull. 1986, 34, 2018. [Google Scholar] [CrossRef]
- Mallory, F.B.; Mallory, C.W. Photocyclization of stilbenes and related molecules. Org. React. 1984, 30. [Google Scholar] [CrossRef]
- Shimoji, Y.; Hashimoto, T.; Furukawa, Y.; Yanagisawa, H. Intramolecular Diels-Alder reaction of 3-formimidoylindoles: Synthesis of fused pyridoindole compounds. Heterocycles 1993, 36, 123. [Google Scholar] [CrossRef]
- Bradbury, R.H. Heterocyclic Compounds Useful as Angiotensin II Antagonists. EP Patent 539066 A1, 28 April 1993. [Google Scholar]
- O’Callaghan, C.N.; McMurry, T.B.H.; O’Brien, J.E. The formation of polyheterocyclic systems by the reaction of 2-oxo-2H-1-benzopyran-3-carboxamide and related compounds with active methylene compounds. J. Chem. Res. Synopses 1995, 27, 490. [Google Scholar] [CrossRef]
- Shao, C. Preparation of tetracyclic compounds for treatment of infection. CN Patent 108658986, 28 March 2017. [Google Scholar]
- Norman, M.; Wang, H.; Rzasa, R.; Zhong, W.; Nguyen, T.; Kaller, M. Preparation of Thiazolyl Substituted Quinolinones for Treating Cell Proliferative Disorders, Neurological Disorders and Apoptosis. WO Patent 2003066630 A2, 14 August 2003. [Google Scholar]
- Czuba, W.; Kowalska, T.; Kowalski, P. Synthesis and amination of 2,4-dibromo-1,6-naphthyridine. Pol. J. Chem. 1978, 52, 2369. [Google Scholar]
- Singh, B.; Lesher, G.Y.; Pluncket, K.C.; Pagani, E.D.; Bode, D.C.; Bentley, R.G.; Connell, M.J.; Hamel, L.T.; Silver, P.J. Novel cAMP PDE III inhibitors: 1,6-naphthyridin-2(1H)-ones. J. Med. Chem. 1992, 35, 4858. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Kang, S.A.; Thoreen, C.C.; Hur, W.; Choi, H.G.; Waller, D.L.; Sim, T.; Sabatini, D.M.; Gray, N.S. Discovery and optimization of potent and selective benzonaphthyridinone analogs as small molecule mTOR inhibitors with improved mouse microsome stability. Bioorg. Med. Chem. Lett. 2011, 21, 4036–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedeva, M.I.; Tugusheva, N.Z.; Alekseeva, L.M.; Evstratova, M.I.; Kiselev, S.S.; Chernyshev, V.V.; Avramenko, G.V.; Granik, V.G. Substituted 3-and 5-formylpyridin-2-ones in the synthesis of 1-aryl-1,6-naphthyridinone derivatives. Russ. Chem. Bull. 2009, 58, 2336–2346. [Google Scholar] [CrossRef]
- Cywin, C.L.; Zhao, B.-P.; McNeil, D.W.; Hrapchak, M.; Prokopowicz, A.S.; Goldberg, D.R.; Morwick, T.M.; Gao, A.; Jakes, S.; Kashem, M.; et al. Discovery and SAR of novel Naphthyridines as potent inhibitors of spleen tyrosine kinase (SYK). Bioorg. Med. Chem. Lett. 2003, 13, 1415–1418. [Google Scholar] [CrossRef]
- Fayed, A.A.; Bahashwan, S.A.; Yousif, M.N.M.; El Shafey, H.M.; Amr, A.E.; Yousif, N.M.; Shadid, K.A. Synthesis and Antiproliferative Activity of Some Newly Synthesized Pyrazolopyridine Derivatives. Russ. J. Gen. Chem. 2019, 89, 1209–1217. [Google Scholar] [CrossRef]
- Achutha, A.S.; Pushpa, V.L.; Manoj, K.B. Comparative molecular docking studies of phytochemicals as Jak2 inhibitors using Autodock and ArgusLab. Mater. Today Proc. 2021, 41, 711–716. [Google Scholar] [CrossRef]
- Siu, T.; Kozina, E.S.; Jung, J.; Rosenstein, C.; Mathur, A.; Altman, M.D.; Chan, G.; Xu, L.; Bachman, E.; Mo, J.-R.; et al. The discovery of tricyclic pyridone JAK2 inhibitors. Part 1: Hit to lead. Bioorg. Med. Chem. Lett. 2010, 20, 7421–7425. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.; Dexheimer, T.S.; Pommier, Y.; Cushman, M. Design, Synthesis, and Evaluation of Dibenzo[c,h][1,6]naphthyridines as Topoisomerase I Inhibitors and Potential Anticancer Agents. J. Med. Chem. 2010, 53, 8716–8726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kousy, S.M.; Mohareb, R.M.; Riad, M.; Elnagdi, M.H. Studies with polyfunctionally substituted heteroaromatic compounds: Synthesis of some new polyfunctionally substituted pyrido[3,2-c]pyridines and pyrido[2,3-c]pyridazines. Pak. J. Sci. Ind. Res. 1998, 41, 81–85. [Google Scholar]
- Daboun, H.A.; Abdou, S.E.; Khader, M.M. Reactions with activated nitriles: Some new approaches to the synthesis of pyridine derivatives. Arch. Pharm. 1983, 316, 564. [Google Scholar] [CrossRef]
- Maxfield, E.K.; Love, A.; Cotter, M.A.; Cameron, N.E. Nerve function and regeneration in diabetic rats: Effects of ZD-7155, an AT1 receptor antagonist. Am. J. Physiol. 1995, 269, e530. [Google Scholar] [CrossRef]
- Zhang, H.; Unal, H.; Gati, C.; Han, G.W.; Liu, W.; Zatsepin, N.A.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; et al. Structure of the Angiotensin Receptor Revealed by Serial Femtosecond Crystallography. Cell 2015, 161, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Dotsenko, V.V.; Ismiev, A.I.; Khrustaleva, A.N.; Frolov, K.A.; Krivokolysko, S.G.; Chigorina, E.A.; Snizhko, A.P.; Gromenko, V.M.; Bushmarinov, I.S.; Askerov, R.K.; et al. Synthesis, structure, and reactions of (4-aryl-3-cyano-6-oxopiperidin-2-ylidene)malononitriles. Chem. Heterocycl. Compd. 2016, 52, 473–483. [Google Scholar] [CrossRef]
- Clark, J.D.; Davis, J.M.; Favor, D.; Fay, L.K.; Franklin, L.; Henegar, K.E.; Johnson, D.S.; Nichelson, B.J.; Ou, L.; Repine, J.T.; et al. Preparation of [1,8]naphthyridin-2-ones and Related Compounds for the Treatment of Schizophrenia, Other Mental Disorders, and Central Nervous System Disorders. US20050043309 A1, 24 February 2005. [Google Scholar]
- Whyte, A.; Olson, M.E.; Lautens, M. Palladium-Catalyzed, Norbornene-Mediated, ortho-Amination ipso-Amidation: Sequential C-N Bond Formation. Org. Lett. 2018, 20, 345–348. [Google Scholar] [CrossRef]
- Thompson, A.M.; Rewcastle, G.W.; Boushelle, S.L.; Hartl, B.G.; Kraker, A.J.; Lu, G.H.; Batley, B.L.; Panek, R.L.; Showalter, H.D.H.; Denny, W.A. Synthesis and Structure-Activity Relationships of 7-Substituted 3-(2,6-Dichlorophenyl)-1,6-naphthyridin-2(1H)-ones as Selective Inhibitors of pp60c-src. J. Med. Chem. 2000, 43, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Satyanarayana, M.; Cheng, L.; Liu, A.; Tsai, Y.-C.; Liu, L.F.; LaVoie, E.J. Synthesis of N-substituted 5-[2-(N-alkylamino)ethyl]dibenzo[c,h][1,6]naphthyridines as novel topoisomerase I-targeting antitumor agents. Bioorg. Med. Chem. 2008, 16, 9295–9301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, M.S.A.; Griggs, B.; Cushman, M. Synthesis of Benzo[1,6]naphthyridinones Using the Catellani Reaction. Org. Lett. 2018, 20, 5228–5232. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, P.; Yang, Y.; Hu, L.; Wang, P.; Jia, S.; Qian, Y. Transition Metal Complex Used as Phosphorescent Material, its Preparation Method and Application in Organic Electroluminescent Device. CN Patent 108690092 A, 23 October 2018. [Google Scholar]
- Alam, M.; Beck, H.P.; Dillon, M.P.; Gonzalez-Lopez, M.; Rico, A.C.; Sutton, J.C., Jr. Preparation of Heteroaryl Amides as AhR Modulators. WO Patent 2019018562 A1, 24 January 2019. [Google Scholar]
- Ketcham, J.M.; Burns, A.C.; Marx, M.A. Naphthyridine Derivatives as PRC2 Inhibitors and Their Preparation. WO Patent 2020219448 A1, 29 October 2020. [Google Scholar]
- Barsy, M.A.; Khalafallah, A.K.; Hassan, M.E.; Rezk, A.A. Successful approach for the synthesis of newly fused heterocyclic compounds incorporating phenylperinaphthenone and naphthyridine derivatives. Heterocycl. Commun. 2000, 6, 339–344. [Google Scholar] [CrossRef]
- Deng, H.; Yu, H.; Chen, Z.; Xu, Y. Process for Preparation of FGFR Inhibitor and Application Thereof. WO Patent 2020052349 A1, 19 March 2020. [Google Scholar]
- Chianelli, D.; Cow, C.; He, Y.; Jiang, S.; Li, X.; Liu, X.; Liu, Z.; Loren, J.; Molteni, V.; Nabakka, J.; et al. Naphthyridinone compositions and methods for modulating c-kit and PDGFR receptors and their preparation. WO Patent 2008051757 A1, 2 May 2008. [Google Scholar]
- Yang, F.; Deng, H.; Ying, H.; Yu, H.; Chen, Z.; Xu, Y. Preparation of FGFR4 kinase inhibitors useful for the treatment of cancer. WO Patent 2018113584 A1, 28 June 2018. [Google Scholar]
- Patterson, A.V.; Smaill, J.B.; Ashoorzadeh, A.; Guise, C.P.; Squire, C.J.; Gamage, S.A.; Abbattista, M.R.; Bull, M.R.; Grey, A.C.; Li, X.; et al. Preparation of Substituted Pyrimidopyrimidinones as FGFR Kinase Inhibitors. WO Patent 2018160076 A1, 7 September 2018. [Google Scholar]
- Xu, Z.; Hu, L.; Ding, C.Z.; Chen, S. Preparation of Pyrazine Derivatives as Cdk4/6 Inhibitors for Treatment of Cancer. WO Patent 2018108167 A1, 21 June 2018. [Google Scholar]
- Zeng, Q.; Faris, M.; Mollard, A.; Warner, S.L.; Flynn, G.A. Preparation of Heteroarylaminonaphthyridinone Derivatives for Use as Multiple Kinase Pathway Inhibitors. WO Patent 2014052365 A1, 3 April 2014. [Google Scholar]
- Bhattacharya, S.K.; Behenna, D.C.; Cameron, K.O.; Chen, P.; Curto, J.M.; Freeman-Cook, K.D.; Jalaie, M.; Kania, R.S.; Lian, Y.; Nair, S.K.; et al. Anti-Proliferative Agents for Treating Pulmonary Arterial Hypertension (PAH). WO Patent 2020212865 A1, 22 October 2020. [Google Scholar]
- Barvian, M.R.; Denny, W.A.; Dobrusin, E.M.; Hamby, J.M.; Showalter, H.D.H.; Thompson, A.M.; Winters, R.T.; Wu, Z. Preparation of Naphthyridinones as Protein Tyrosine Kinase and Cyclin Dependant Kinase Inhibitors. WO Patent 9909030 A1, 25 February 1999. [Google Scholar]
- Cheng, C.-C.; Yan, S.-J. The Friedlander synthesis of quinolines. Org. React. 1982, 28. [Google Scholar] [CrossRef]
- Dunn, A.D. Synthesis of novel naphthyridines. Z. Chem. 1990, 30, 20. [Google Scholar] [CrossRef]
- Singh, B.; Bacon, E.R.; Lesher, G.Y.; Robinson, S.; Pennock, P.O.; Bode, D.C.; Pagani, E.D.; Bentley, R.G.; Connell, M.J. Novel and Potent Adenosine 3′,5′-Cyclic Phosphate Phosphodiesterase III Inhibitors: Thiazolo[4,5-b][1,6]naphthyridin-2-ones. J. Med. Chem. 1995, 38, 2546. [Google Scholar] [CrossRef]
- Savarin, C.G.; Murry, J.A.; Dormer, P.G. An Expedient Synthesis of Highly Functionalized Naphthyridones and Quinolines from a Common N-Aryl Pyridinone Template. Org. Lett. 2002, 4, 2071–2074. [Google Scholar] [CrossRef]
- Di Pietro, O.; Vicente-Garcia, E.; Taylor, M.C.; Berenguer, D.; Viayna, E.; Lanzoni, A.; Sola, I.; Sayago, H.; Riera, C.; Fisa, R.; et al. Multicomponent reaction-based synthesis and biological evaluation of tricyclic heterofused quinolines with multi-trypanosomatid activity. Eur. J. Med. Chem. 2015, 105, 120–137. [Google Scholar] [CrossRef]
- Lin, S.; Han, F.; Liu, P.; Tao, J.; Zhong, X.; Liu, X.; Yi, C.; Xu, H. Identification of novel 7-amino-5-methyl-1,6-naphthyridin-2(1H)-one derivatives as potent PI3K/mTOR dual inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 790–793. [Google Scholar] [CrossRef]
- Yoshida, K.; Perich, R.; Casley, D.J.; Johnston, C.I. Hypotensive effect of ZD7155, an angiotensin II receptor antagonist, parallels receptor occupancy in two-kidney, one-clip Goldblatt hypertensive rats. J. Hypertens. 1998, 16, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Junggren, I.-L.; Zhao, X.; Sun, X.; Hedner, T. Comparative cardiovascular effects of the angiotensin II type 1 receptor antagonists ZD 7155 and losartan in the rat. J. Pharm. Pharmacol. 1996, 48, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Ripretinib: First Approval. Drugs 2020, 80, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Bauer, S.; Blay, J.-Y.; Choucair, K.; Gelderblom, H.; George, S.; Schoeffski, P.; von Mehren, M.; Zalcberg, J.; Achour, H.; et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Futur. Oncol. 2020, 16, 4251–4264. [Google Scholar] [CrossRef] [Green Version]
- Blay, J.-Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schoffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef]

















| R 1 | Structures 14 (%) | References 1 | Structures 13 (%) | References |
|---|---|---|---|---|
| H | 51.86 | [12,13] | 35.95 | [14,15] |
| Me | 1.65 | [16,17] | 10.47 | [18,19] |
| Alkyl | 5.77 | [20,21] | 7.29 | [22,23] |
| Carbocycle | 2.16 | [20,24] | 2.76 | [25,26] |
| Ph | 15.16 | [24,27] | 17.47 | [28,29] |
| Heterocycle | 4.12 | [30,31] | 4.29 | [32,33] |

| Substituent | R5 | R7 | ||
|---|---|---|---|---|
| Structures (%) | References | Structures (%) | References | |
| H | 78.19 | 49 [36,40] | 65.87 | 42 [12,41] |
| C | 1.05 | 56 [39,42] | 25.65 | 72 [38,43] |
| N | 0.77 | 9 [8,35] | 2.28 | 12 [34,35] |
| O | 5.27 | 9 [37,44] | 5.62 | 7 [8,40] |
| X | 14.69 | 11 [8,9] | 0.47 | 11 [9,34] |

| Substituent | R5 | R7 | ||
|---|---|---|---|---|
| Structures (%) | References | Structures (%) | References | |
| H | 66.59 | 808 [28,46] | 33.98 | 246 [11,47] |
| C | 20.69 | 113 [39,48] | 43.25 | 614 [39,49] |
| N | 3.92 | 19 [50,51] | 16.34 | 125 [52,53] |
| O | 8.25 | 41 [54,55] | 4.20 | 29 [56,57] |
| Compounds 14 | Compounds 13 | ||
|---|---|---|---|
| Index Term | Frequency | Index Term | Frequency |
| Angiotensin AT1 receptors | 19 | Antitumor agents | 281 |
| Cardiovascular agents | 18 | Signal transduction | 235 |
| Animals | 17 | Animals | 205 |
| Antihypertensives | 16 | Neoplasm | 163 |
| Angiotensin II receptor antagonists | 15 | Protein phosphorylation | 135 |
| Combination chemotherapy | 13 | Target of rapamycin complex 1 | 130 |
| Heart failure | 13 | TOR Serine-Threonine Kinases | 129 |
| Antidiabetic agents | 10 | Autophagy | 120 |
| Antitumor agents | 10 | Cell proliferation | 120 |
| Antiarthritics | 5 | Lung neoplasm | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveras, J.M.; Puig de la Bellacasa, R.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1,6-Naphthyridin-2(1H)-ones: Synthesis and Biomedical Applications. Pharmaceuticals 2021, 14, 1029. https://doi.org/10.3390/ph14101029
Oliveras JM, Puig de la Bellacasa R, Estrada-Tejedor R, Teixidó J, Borrell JI. 1,6-Naphthyridin-2(1H)-ones: Synthesis and Biomedical Applications. Pharmaceuticals. 2021; 14(10):1029. https://doi.org/10.3390/ph14101029
Chicago/Turabian StyleOliveras, Juan Marcos, Raimon Puig de la Bellacasa, Roger Estrada-Tejedor, Jordi Teixidó, and José I. Borrell. 2021. "1,6-Naphthyridin-2(1H)-ones: Synthesis and Biomedical Applications" Pharmaceuticals 14, no. 10: 1029. https://doi.org/10.3390/ph14101029