Remyelination-Promoting DNA Aptamer Conjugate Myaptavin-3064 Binds to Adult Oligodendrocytes In Vitro
Abstract
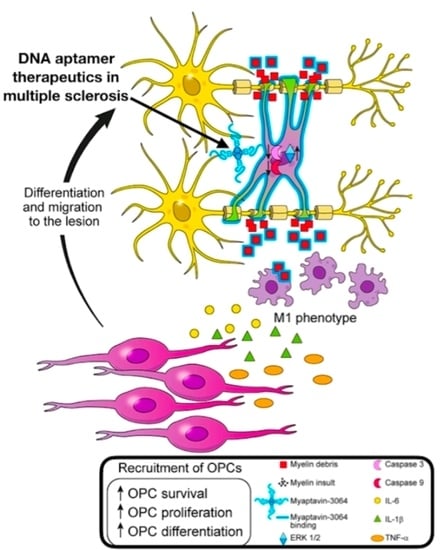
:1. Introduction
2. Results
2.1. Characterization of HOG Cells in Culture
2.2. Specific Binding of Myaptavin-3064 to HOG Cells
2.3. Myaptavin-3064 Cellular Targets
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Antibodies
4.3. Preparation of Aptamer Complex
4.4. Animals
4.5. Cell Culture
4.6. Isolation of Mouse Mixed Cortical Cells
4.7. Adult Rat Mixed Glial Culture
4.8. Flow Cytometry
4.9. Immunocytochemistry
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rotstein, D.L.; Chen, H.; Wilton, A.S.; Kwong, J.C.; Marrie, R.A.; Gozdyra, P.; Krysko, K.M.; Kopp, A.; Copes, R.; Tu, K. Temporal trends in multiple sclerosis prevalence and incidence in a large population. Neurology 2018, 90, e1435–e1441. [Google Scholar] [CrossRef] [PubMed]
- Loleit, V.; Biberacher, V.; Hemmer, B. Current and future therapies targeting the immune system in multiple sclerosis. Curr. Pharm. Biotechnol. 2014, 15, 276–296. [Google Scholar] [CrossRef] [PubMed]
- Coret, F.; Perez-Miralles, F.C.; Gascon, F.; Alcala, C.; Navarre, A.; Bernad, A.; Bosca, I.; Escutia, M.; Gil-Perotin, S.; Casanova, B. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult. Scler. J. Exp. Transl. Clin. 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal-Bianco, A.; Lassmann, H.; Frischer, J.M.; Sorensen, P.S.; Bramow, S.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef] [Green Version]
- Warrington, A.E.; Asakura, K.; Bieber, A.J.; Ciric, B.; Van Keulen, V.; Kaveri, S.V.; Kyle, R.A.; Pease, L.R.; Rodriguez, M. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2000, 97, 6820–6825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Nastasijevic, B.; Wright, B.R.; Smestad, J.; Warrington, A.E.; Rodriguez, M.; Maher, L.J., 3rd. Remyelination induced by a DNA aptamer in a mouse model of multiple sclerosis. PLoS ONE 2012, 7, e39595. [Google Scholar] [CrossRef] [Green Version]
- Batool, S.; Argyropoulos, K.V.; Azad, R.; Okeoma, P.; Zumrut, H.; Bhandari, S.; Dekhang, R.; Mallikaratchy, P.R. Dimerization of an aptamer generated from Ligand-guided selection (LIGS) yields a high affinity scaffold against B-cells. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 232–240. [Google Scholar] [CrossRef]
- Riccardi, C.; Russo Krauss, I.; Musumeci, D.; Morvan, F.o.; Meyer, A.; Vasseur, J.-J.; Paduano, L.; Montesarchio, D. Fluorescent thrombin binding aptamer-tagged nanoparticles for an efficient and reversible control of thrombin activity. ACS Appl. Mater. Interfaces 2017, 9, 35574–35587. [Google Scholar] [CrossRef]
- Zavyalova, E.G.; Legatova, V.A.; Alieva, R.S.; Zalevsky, A.O.; Tashlitsky, V.N.; Arutyunyan, A.M.; Kopylov, A.M. Putative mechanisms underlying high inhibitory activities of bimodular DNA aptamers to thrombin. Biomolecules 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Moccia, F.; Riccardi, C.; Musumeci, D.; Leone, S.; Oliva, R.; Petraccone, L.; Montesarchio, D. Insights into the G-rich VEGF-binding aptamer V7t1: When two G-quadruplexes are better than one! Nucleic Acids Res. 2019, 47, 8318–8331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perschbacher, K.; Smestad, J.A.; Peters, J.P.; Standiford, M.M.; Denic, A.; Wootla, B.; Warrington, A.E.; Rodriguez, M.; Maher, L.J., 3rd. Quantitative PCR analysis of DNA aptamer pharmacokinetics in mice. Nucleic Acid Res. 2015, 25, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.D.; Rhodes, D.G.; Burgess, D.J. DNA-based therapeutics and DNA delivery systems: A comprehensive review. Aaps J. 2005, 7, E61–E77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini Shamili, F.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Mahmoudi, M.; Kalantari, M.; Taghdisi, S.M.; Ramezani, M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J. Control. Release 2019, 299, 149–164. [Google Scholar] [CrossRef]
- Duncan, I.D.; Brower, A.; Kondo, Y.; Curlee, J.F., Jr.; Schultz, R.D. Extensive remyelination of the CNS leads to functional recovery. Proc. Natl. Acad. Sci. USA 2009, 106, 6832–6836. [Google Scholar] [CrossRef] [Green Version]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin-From mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Franklin, R.J.; Gallo, V. The translational biology of remyelination: Past, present, and future. Glia 2014, 62, 1905–1915. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef]
- Nait-Oumesmar, B.; Picard-Riera, N.; Kerninon, C.; Decker, L.; Seilhean, D.; Höglinger, G.U.; Hirsch, E.C.; Reynolds, R.; Baron-Van Evercooren, A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 4694–4699. [Google Scholar] [CrossRef] [Green Version]
- Crawford, A.H.; Tripathi, R.B.; Foerster, S.; McKenzie, I.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D.; Franklin, R.J. Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination. Am. J. Pathol. 2016, 186, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisén, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Gensert, J.M.; Goldman, J.E. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 1997, 19, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Fereidan-Esfahani, M.; Nayfeh, T.; Warrington, A.; Howe, C.L.; Rodriguez, M. IgM Natural Autoantibodies in Physiology and the Treatment of Disease. Methods Mol. Biol 2019, 1904, 53–81. [Google Scholar] [CrossRef]
- Kotter, M.R.; Li, W.W.; Zhao, C.; Franklin, R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 2006, 26, 328–332. [Google Scholar] [CrossRef]
- Plemel, J.R.; Manesh, S.B.; Sparling, J.S.; Tetzlaff, W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia 2013, 61, 1471–1487. [Google Scholar] [CrossRef]
- Zorina, Y.; Stricker, J.; Caggiano, A.O.; Button, D.C. Human IgM antibody rHIgM22 promotes phagocytic clearance of myelin debris by microglia. Sci. Rep. 2018, 8, 9392. [Google Scholar] [CrossRef] [Green Version]
- Lodge, P.A.; Sriram, S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. J. Leukoc. Biol. 1996, 60, 502–508. [Google Scholar] [CrossRef]
- Barres, B.A.; Schmid, R.; Sendnter, M.; Raff, M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 1993, 118, 283–295. [Google Scholar] [PubMed]
- Kahn, M.A.; De Vellis, J. Regulation of an oligodendrocyte progenitor cell line by the interleukin-6 family of cytokines. Glia 1994, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; Ferrario, M.; Dreano, M.; Garotta, G.; Spano, P.; Pizzi, M. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Mol. Cell. Neurosci. 2002, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Arnett, H.A.; Mason, J.; Marino, M.; Suzuki, K.; Matsushima, G.K.; Ting, J.P. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001, 4, 1116–1122. [Google Scholar] [CrossRef]
- Cripps, A.W.; Neoh, S.H.; Smart, I.J. Isolation of human IgA and IgM from normal serum using polyethylene glycol precipitation and affinity chromatography. J. Immunol. Methods 1983, 57, 197–204. [Google Scholar] [CrossRef]
- Sauer, B.M.; Schmalstieg, W.F.; Howe, C.L. Axons are injured by antigen-specific CD8(+) T cells through a MHC class I- and granzyme B-dependent mechanism. Neurobiol. Dis. 2013, 59, 194–205. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fereidan-Esfahani, M.; Yue, W.Y.; Wilbanks, B.; Johnson, A.J.; Warrington, A.E.; Howe, C.L.; Rodriguez, M.; Maher, L.J., III. Remyelination-Promoting DNA Aptamer Conjugate Myaptavin-3064 Binds to Adult Oligodendrocytes In Vitro. Pharmaceuticals 2020, 13, 403. https://doi.org/10.3390/ph13110403
Fereidan-Esfahani M, Yue WY, Wilbanks B, Johnson AJ, Warrington AE, Howe CL, Rodriguez M, Maher LJ III. Remyelination-Promoting DNA Aptamer Conjugate Myaptavin-3064 Binds to Adult Oligodendrocytes In Vitro. Pharmaceuticals. 2020; 13(11):403. https://doi.org/10.3390/ph13110403
Chicago/Turabian StyleFereidan-Esfahani, Mahboubeh, Wei Ying Yue, Brandon Wilbanks, Aaron J. Johnson, Arthur E. Warrington, Charles L. Howe, Moses Rodriguez, and Louis J. Maher, III. 2020. "Remyelination-Promoting DNA Aptamer Conjugate Myaptavin-3064 Binds to Adult Oligodendrocytes In Vitro" Pharmaceuticals 13, no. 11: 403. https://doi.org/10.3390/ph13110403