Electrochemical Synthesis of Reduced Graphene Oxide/Gold Nanoparticles in a Single Step for Carbaryl Detection in Water
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Materials
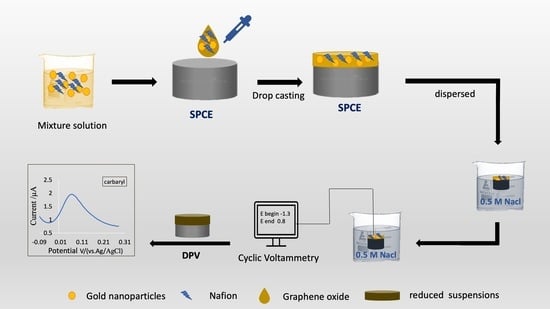
2.2. Solutions Preparation
2.3. Nanocomposite-Modified Electrode Preparation
2.4. Physical and Electrochemical Analysis
2.5. Real Sample Preparation
2.6. Traditional Capillary Electrophoresis with UV Detector (CE-UV)
2.7. Sample Preparation and Solid Phase Extraction (SPE)
3. Results and Discussion
3.1. Electrode Activation
3.2. Optimization of Nanocomposite Sensor for Carbaryl Detection
3.3. Characterization of Activated and Nanocomposite-Modified SPC Electrodes
3.4. Optimization Study
3.5. Calibration, Selectivity, Stability and Reproducibility
3.6. Real Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umina, P.A.; McDonald, G.; Maino, J.; Edwards, O.; Hoffmann, A.A. Escalating Insecticide Resistance in Australian Grain Pests: Contributing Factors, Industry Trends and Management Opportunities. Pest Manag. Sci. 2019, 75, 1494–1506. [Google Scholar] [CrossRef]
- Kanyika-Mbewe, C.; Thole, B.; Makwinja, R.; Kaonga, C.C. Monitoring of Carbaryl and Cypermethrin Concentrations in Water and Soil in Southern Malawi. Environ. Monit. Assess. 2020, 192, 595. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.U.; Halimoon, N.; Johar, W.L.W.; Shukor, M.Y.A. An Overview on Biodegradation of Carbamate Pesticides by Soil Bacteria. Pertanika J. Sci. Technol. 2019, 27, 547–563. [Google Scholar]
- Firdoz, S.; Ma, F.; Yue, X.; Dai, Z.; Kumar, A.; Jiang, B. A Novel Amperometric Biosensor Based on Single Walled Carbon Nanotubes with Acetylcholine Esterase for the Detection of Carbaryl Pesticide in Water. Talanta 2010, 83, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, J.Y.; Kim, B. Accurate Determination of Carbaryl, Carbofuran and Carbendazim in Vegetables by Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry. Chromatographia 2021, 84, 27–35. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Yilmaz, E.; Habila, M.A.; Alhenaki, B.; Soylak, M.; Ahmed, A.Y.B.H.; Alabdullkarem, E.A. Development of Combined-Supramolecular Microextraction with Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Procedures for Ultra-Trace Analysis of Carbaryl in Water, Fruits and Vegetables. Int. J. Environ. Anal. Chem. 2020, 102, 1491–1501. [Google Scholar] [CrossRef]
- Nagasawa, K.; Uchiyama, H.; Ogamo, A.; Shinozuka, T. Gas Chromatographic Determination of Microamounts of Carbaryl and 1-Napththol in Natural Water as Sources of Water Supplies. J. Chromatogr. A 1977, 144, 77–84. [Google Scholar] [CrossRef]
- Xiaona, J.; Lin, F.; Ren, Z.; Tian, X.; Zuo, Y.; Bian, D. Detection of the Carbaryl in Wastewater by HPLC-DAD. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 25–26 September 2021; Volume 835, p. 012004. [Google Scholar] [CrossRef]
- Ren, Z.; Zhou, X.; Gao, X.; Tan, Y.; Chen, H.; Tan, S.; Liu, W.; Tong, Y.; Chen, C. Rapid Detection of Carbamate Pesticide Residues Using Microchip Electrophoresis Combining Amperometric Detection. Anal. Bioanal. Chem. 2021, 413, 3017–3026. [Google Scholar] [CrossRef]
- Apilux, A.; Ayudhya, C.I.N.; Tantimongcolwat, T.; Prachayasittikul, V. Paper-Based Acetylcholinesterase Inhibition Assay Combining a Wet System for Organophosphate and Carbamate Pesticides Detection. EXCLI J. 2015, 14, 307–319. [Google Scholar] [CrossRef]
- Romanholo, P.V.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic Electrochemical Sensors: New Horizons and Challenges in Biosensing Applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Fahimi-Kashani, N.; Hormozi-nezhad, M.R. A Ratiometric Fluorescence Nanoprobe Using CdTe QDs for Fast Detection of Carbaryl Insecticide in Apple. Talanta 2021, 221, 121467. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Yang, Y.; Zhang, Y.; Wang, Y.; Chen, X. Ratiometric Strategy for Electrochemical Sensing of Carbaryl Residue in Water and Vegetable Samples. Sensors 2020, 20, 1524. [Google Scholar] [CrossRef] [Green Version]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct Electrochemical Determination of Carbaryl Using a Multi-Walled Carbon Nanotube/Cobalt Phthalocyanine Modified Electrode. Talanta 2009, 79, 1406–1411. [Google Scholar] [CrossRef]
- Codognoto, L.; Tanimoto, S.T.; Pedrosa, V.A.; Suffredini, H.B.; Machado, S.A.S.; Avaca, L.A. Electroanalytical Determination of Carbaryl in Natural Waters on Boron Doped Diamond Electrode. Electroanalysis 2006, 18, 253–258. [Google Scholar] [CrossRef]
- Lv, H.; Yang, L.; Zhou, Y.; Qu, R.; Xu, Y.; Shang, S.; Hui, N. Non-Enzymatic Electrochemical Sensors Based on Conducting Polymer Hydrogels for Ultrasensitive Carbaryl Pesticide Detection. J. Electrochem. Soc. 2021, 168, 047506. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.B.; Inamuddin, I.; Mishra, A.K. Carbon-Based Nanomaterials for Remediation of Organic and Inorganic Pollutants from Wastewater. A Review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chan, K.K.; Tjin, S.C.; Yong, K.T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors 2020, 20, 2046. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; He, S.; Nag, A.; Wong, J.W.C. A Review of the Use of Carbon Nanotubes and Graphene-Based Sensors for the Detection of Aflatoxin M1 Compounds in Milk. Sensors 2021, 21, 3602. [Google Scholar] [CrossRef]
- Nag, A.; Alahi, M.E.E.; Mukhopadhyay, S.C.; Liu, Z. Multi-Walled Carbon Nanotubes-Based Sensors for Strain Sensing Applications. Sensors 2021, 21, 1261. [Google Scholar] [CrossRef]
- Łoś, S.; Fabisiak, K.; Paprocki, K.; Szybowicz, M.; Dychalska, A. The Undoped Polycrystalline Diamond Film—Electrical Transport Properties. Sensors 2021, 21, 6113. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Casillas, N.; Astudillo-Sánchez, P.D.; Flores-Moreno, R. Role of Defects on Regioselectivity of Nano Pristine Graphene. J. Phys. Chem. A 2016, 120, 9101–9108. [Google Scholar] [CrossRef] [PubMed]
- Chintalapudi, K.; Pannem, R.M.R. An Intense Review on the Performance of Graphene Oxide and Reduced Graphene Oxide in an Admixed Cement System. Constr. Build. Mater. 2020, 259, 120598. [Google Scholar] [CrossRef]
- Maharubin, S.; Zhang, X.; Zhu, F.; Zhang, H.C.; Zhang, G.; Zhang, Y. Synthesis and Applications of Semiconducting Graphene. J. Nanomater. 2016, 2016, 6375962. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, G.K. Pre-Post Redox Electron Transfer Regioselectivity at the Alanine Modified Nano Graphene Electrode Interface. Chem. Phys. Lett. 2022, 789, 139295. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Flores-Moreno, R. Quantum Chemical Study of Triton X-100 Modified Graphene Surface. Electrochim. Acta 2017, 248, 225–231. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Flores-Moreno, R. Regioselectivity in Hexagonal Boron Nitride Co-Doped Graphene. New J. Chem. 2018, 42, 18913–18918. [Google Scholar] [CrossRef]
- Kudur Jayaprakash, G.; Swamy, B.E.K.; Casillas, N.; Flores-Moreno, R. Analytical Fukui and Cyclic Voltammetric Studies on Ferrocene Modified Carbon Electrodes and Effect of Triton X-100 by Immobilization Method. Electrochim. Acta 2017, 258, 1025–1034. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A Comprehensive Review of Applications of Magnetic Graphene Oxide Based Nanocomposites for Sustainable Water Purification. J. Environ. Manag. 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Chen, T.; Ma, Y.; Guo, Q.; Yang, M.; Xia, H. A Facile Sol-Gel Route to Prepare Functional Graphene Nanosheets Anchored with Homogeneous Cobalt Sulfide Nanoparticles as Superb Sodium-Ion Anodes. J. Mater. Chem. A 2017, 5, 3179–3185. [Google Scholar] [CrossRef]
- Zeng, X.; Teng, J.; Yu, J.; Tan, A.; Fu, D.; Zhang, H. Fabrication of Homogeneously Dispersed Graphene/Al Composites by Solution Mixing and Powder Metallurgy. Int. J. Miner. Metall. Mater. 2018, 25, 102–109. [Google Scholar] [CrossRef]
- Ikram, M.; Raza, A.; Imran, M.; Ul-Hamid, A.; Shahbaz, A.; Ali, S. Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (RGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment. Nanoscale Res. Lett. 2020, 15, 95. [Google Scholar] [CrossRef]
- Nagaraju, P.; Alsalme, A.; Alkathiri, A.M.; Jayavel, R. Rapid Synthesis of WO3/Graphene Nanocomposite via in-Situ Microwave Method with Improved Electrochemical Properties. J. Phys. Chem. Solids 2018, 120, 250–260. [Google Scholar] [CrossRef]
- Xu, T.; Chen, J.; Yuan, W.; Liu, Y.; Sun, Y.; Wu, H.; Zhou, X. Self-Assembly Synthesis of Silver Nanowires/ Graphene Nanocomposite and Its Effects on the Performance of Electrically Conductive Adhesive. Materials 2018, 11, 2028. [Google Scholar] [CrossRef] [Green Version]
- Razmi, H.; Ezzati, L.; Khorablou, Z. Direct Electrochemical Synthesis of Graphene Oxide/Cobalt Oxide Nanocomposite on Pencil Graphite Electrode for Highly Sensitive and Selective Detection of Insulin in Pharmaceutical Samples. J. Electrochem. Soc. 2019, 166, B961–B968. [Google Scholar] [CrossRef]
- Dutta, V.; Singh, P.; Shandilya, P.; Sharma, S.; Raizada, P.; Saini, A.K.; Gupta, V.K.; Hosseini-Bandegharaei, A.; Agarwal, S.; Rahmani-Sani, A. Review on Advances in Photocatalytic Water Disinfection Utilizing Graphene and Graphene Derivatives-Based Nanocomposites. J. Environ. Chem. Eng. 2019, 7, 103132. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and Antibacterial Properties of Gold Nanoparticles: A Review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Amir, M.N.I.; Halilu, A.; Julkapli, N.M.; Ma’amor, A. Gold-Graphene Oxide Nanohybrids: A Review on Their Chemical Catalysis. J. Ind. Eng. Chem. 2020, 83, 1–13. [Google Scholar] [CrossRef]
- Jirasirichote, A.; Punrat, E.; Suea-Ngam, A.; Chailapakul, O.; Chuanuwatanakul, S. Voltammetric Detection of Carbofuran Determination Using Screen-Printed Carbon Electrodes Modified with Gold Nanoparticles and Graphene Oxide. Talanta 2017, 175, 331–337. [Google Scholar] [CrossRef]
- Cao, X.; Hogan, A.; Moore, E. Rapid Separation of Organophosphate Pesticides Using Micellar Electrokinetic Chromatography and Short-End Injection. J. Forensic Sci. 2019, 64, 1213–1220. [Google Scholar] [CrossRef]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Moore, E. A Sensitive Electrochemical Analysis for Cadmium and Lead Based on Nafion-Bismuth Film in a Water Sample. Sens. Bio-Sens. Res. 2021, 34, 100454. [Google Scholar] [CrossRef]
- Wanga, J.; Pedreroa, M.; Sakslund, H.; Hammerichb, O. Electrochemical activation of screen-printed carbon strips. Analyst 1996, 121, 345–350. [Google Scholar] [CrossRef]
- Cui, G.; Yoo, J.H.; Lee, J.S.; Yoo, J.; Uhm, J.H.; Cha, G.S.; Nam, H. Effect of Pre-Treatment on the Surface and Electrochemical Properties of Screen-Printed Carbon Paste Electrodes. Analyst 2001, 126, 1399–1403. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly Activated Screen-Printed Carbon Electrodes by Electrochemical Treatment with Hydrogen Peroxide. Electrochem. Commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- Liu, M.; Pan, D.; Pan, W.; Zhu, Y.; Hu, X.; Han, H.; Wang, C.; Shen, D. In-Situ Synthesis of Reduced Graphene Oxide/Gold Nanoparticles Modified Electrode for Speciation Analysis of Copper in Seawater. Talanta 2017, 174, 500–506. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; Salih, F.E.; el Rhazi, M. Ionic Liquid/Carbon Nanofibers/Bismuth Particles Novel Hybrid Nanocomposite for Voltammetric Sensing of Heavy Metals. J. Environ. Chem. Eng. 2020, 8, 103774. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, L.; Wu, S.; Qin, L.; Kang, S.; Li, X. A Patterned Aluminum/Reduced Graphene Oxide/Silver Sheet for Detection and Degradation of Malachite Green in Water. Sep. Purif. Technol. 2021, 272, 118892. [Google Scholar] [CrossRef]
- Bernalte, E.; Marín Sánchez, C.; Pinilla Gil, E. Gold Nanoparticles-Modified Screen-Printed Carbon Electrodes for Anodic Stripping Voltammetric Determination of Mercury in Ambient Water Samples. Sens. Actuators B Chem. 2012, 161, 669–674. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A Novel Nanocomposite Electrochemical Sensor Based on Green Synthesis of Reduced Graphene Oxide/Gold Nanoparticles Modified Screen Printed Electrode for Determination of Tryptophan Using Response Surface Methodology Approach. Microchem. J. 2020, 154, 104634. [Google Scholar] [CrossRef]
- Analysis, V.; Ii, C. Bismuth Subcarbonate Decorated Reduced Graphene Oxide Nanocomposite for the Sensitive Stripping. Sensors 2020, 20, 6085. [Google Scholar]
- Salih, F.E.; Oularbi, L.; Halim, E.; Elbasri, M.; Ouarzane, A.; el Rhazi, M. Conducting Polymer/Ionic Liquid Composite Modified Carbon Paste Electrode for the Determination of Carbaryl in Real Samples. Electroanalysis 2018, 30, 1847–1856. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; del Carlo, M.; Pepe, A.; Compagnone, D. Nano Carbon Black-Based Screen Printed Sensor for Carbofuran, Isoprocarb, Carbaryl and Fenobucarb Detection: Application to Grain Samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, J.; Wang, M.; Zhang, D.; Chen, J. Electrochemical Nonenzymatic Sensor Based on CoO Decorated Reduced Graphene Oxide for the Simultaneous Determination of Carbofuran and Carbaryl in Fruits and Vegetables. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xiao, B.; Cui, L. Electrochemical Analysis of Carbaryl in Fruit Samples on Graphene Oxide-Ionic Liquid Composite Modified Electrode. J. Food Compos. Anal. 2015, 40, 14–18. [Google Scholar] [CrossRef]
- Salih, F.E.; Achiou, B.; Ouammou, M.; Bennazha, J.; Ouarzane, A.; Younssi, S.A.; el Rhazi, M. Electrochemical Sensor Based on Low Silica X Zeolite Modified Carbon Paste for Carbaryl Determination Application of Modified Electrode by Zeolite to Detection of Carbaryl. J. Adv. Res. 2017, 8, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Pop, A.; Manea, F.; Flueras, A.; Schoonman, J. Simultaneous Voltammetric Detection of Carbaryl and Paraquat Pesticides on Graphene-Modified Boron-Doped Diamond Electrode. Sensors 2017, 17, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Electrode | Charge Transfer Resistance (Rct) (Ω) | Rate Constant (Ket) (cm2) |
|---|---|---|
| Activated SPCE | 0.7 × 102 | 1.1 × 10−1 |
| rGO/NA/SPCE | 0.5 × 102 | 1.2 × 10−1 |
| rGO/AuNPs/NA/SPCE | 0.3 × 102 | 1.3 × 10−1 |
| Sample | Spiked (µM) | rGO/AuNPs/NA/SPCE | CE-UV | ||||
|---|---|---|---|---|---|---|---|
| Found | Recovery% | RSD% | Found | Recovery% | RSD% | ||
| River water | 60.00 | 53.30 | 88.60 | 2.30 | 75.3 | 125.5 | 1.60 |
| 125.00 | 115.56 | 92.45 | 3.10 | 145.3 | 116.24 | 2.5 | |
| Tap water | 60.00 | 67.00 | 111.6 | 2.67 | 59.00 | 98.30 | 1.75 |
| 125.00 | 120 | 96 | 2.25 | 120 | 96 | 1.70 | |
| Electrode | Analyte (Carbaryl) | Linear Range (µM) | LOD (µM) | Sample | Ref. |
|---|---|---|---|---|---|
| Poly-pPDs IL/CPE | Indirect | 0.5–200 | 0.09 | water and fruit | [52] |
| Carbon black nanoparticles/SPE | Indirect | 0.1–100 | 0.048 | food | [53] |
| CoO/rGO/GCE | Indirect | 0.5–200 | 7.5 | fruit and vegetables | [54] |
| GO-IL/GCE | Indirect | 0.1–12 | 0.02 | fruit | [55] |
| Low silica X zeolite modified/CPE | Indirect | 1–100 | 0.3 | tomato | [56] |
| MWCNT/CoPc/GCE | Direct | 0.30−6.61 | 0.005 | river water | [14] |
| GR/BDD | Direct | 1–6 | 0.07 | apple juice | [57] |
| rGO/AuNP/NA/SPCE | Indirect | 0.5–250 | 0.2 | river and tap water | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, I.; Alatawi, H.; Alsefri, S.; Moore, E. Electrochemical Synthesis of Reduced Graphene Oxide/Gold Nanoparticles in a Single Step for Carbaryl Detection in Water. Sensors 2022, 22, 5251. https://doi.org/10.3390/s22145251
Albalawi I, Alatawi H, Alsefri S, Moore E. Electrochemical Synthesis of Reduced Graphene Oxide/Gold Nanoparticles in a Single Step for Carbaryl Detection in Water. Sensors. 2022; 22(14):5251. https://doi.org/10.3390/s22145251
Chicago/Turabian StyleAlbalawi, Ibtihaj, Hanan Alatawi, Samia Alsefri, and Eric Moore. 2022. "Electrochemical Synthesis of Reduced Graphene Oxide/Gold Nanoparticles in a Single Step for Carbaryl Detection in Water" Sensors 22, no. 14: 5251. https://doi.org/10.3390/s22145251