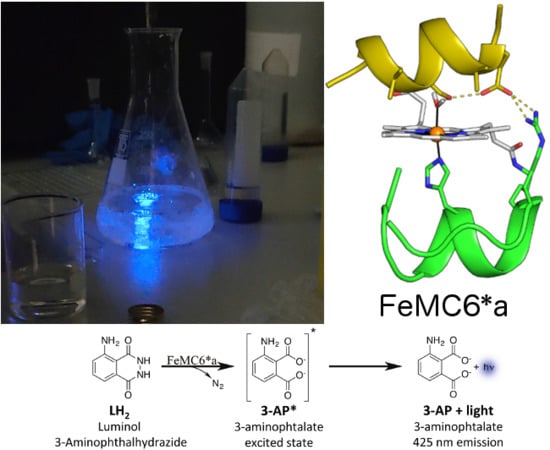
Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Enzyme Synthesis and Purification
2.3. Luminescence Standard Assay
2.4. Steady-State Luminescence Kinetics
2.5. Simulated Advanced Oxidation Process of Thioanisole
3. Results and Discussion
3.1. Assessment of the Artificial Peroxidase Proficiency in Luminol Oxidation
3.2. Effect of pH and Luminol Concentration
3.3. Hydrogen Peroxide Determination
3.4. Sample Analysis and Organic Contaminant Interference Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN-Water Climate Change. UN-Water. Available online: https://www.unwater.org/water-facts/climate-change/ (accessed on 14 January 2020).
- WHO|10 Facts on Climate Change and Health. Available online: https://www.who.int/features/factfiles/climate_change/en/ (accessed on 14 January 2020).
- OHCHR|Special Rapporteur on the Human Rights to Safe Drinking Water and Sanitation. Available online: https://www.ohchr.org/EN/Issues/WaterAndSanitation/SRWater/Pages/SRWaterIndex.aspx (accessed on 14 January 2020).
- Sherchan, S.; Miles, S.; Ikner, L.; Yu, H.-W.; Snyder, S.A.; Pepper, I.L. Near Real-Time Detection of Ecoli in Reclaimed Water. Sensors 2018, 18, 2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Roszak, D.B.; Colwell, R.R. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 1987, 51, 365–379. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Applications of the luminol chemiluminescent reaction in analytical chemistry. Anal. Bioanal. Chem. 2006, 385, 546–554. [Google Scholar] [CrossRef]
- Barni, F.; Lewis, S.W.; Berti, A.; Miskelly, G.M.; Lago, G. Forensic application of the luminol reaction as a presumptive test for latent blood detection. Talanta 2007, 72, 896–913. [Google Scholar] [CrossRef]
- Fernandez-Romero, J.M.; Luque de Castro, M.D. Flow-through optical biosensor based on the permanent immobilization of an enzyme and transient retention of a reaction product. Anal. Chem. 1993, 65, 3048–3052. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Du, J. Bifunctional gold nanoclusters enable ratiometric fluorescence nanosensing of hydrogen peroxide and glucose. Talanta 2019, 197, 599–604. [Google Scholar] [CrossRef]
- Zhao, T.T.; Jiang, Z.W.; Zhen, S.J.; Huang, C.Z.; Li, Y.F. A copper (II)/cobalt (II) organic gel with enhanced peroxidase-like activity for fluorometric determination of hydrogen peroxide and glucose. Microchim. Acta 2019, 186, 168. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Chen, L. Organic-phase biosensors for monitoring phenol and hydrogen peroxide in pharmaceutical antibacterial products. Analyst 1993, 118, 277–280. [Google Scholar] [CrossRef]
- Mulchandani, A.; Rudolph, D.C. Amperometric determination of lipid hydroperoxides. Anal. Biochem. 1995, 225, 277–282. [Google Scholar] [CrossRef]
- Somasundrum, M.; Kirtikara, K.; Tanticharoen, M. Amperometric determination of hydrogen peroxide by direct and catalytic reduction at a copper electrode. Anal. Chim. Acta 1996, 319, 59–70. [Google Scholar] [CrossRef]
- Astuti, Y.; Topoglidis, E.; Cass, A.G.; Durrant, J.R. Direct spectroelectrochemistry of peroxidases immobilised on mesoporous metal oxide electrodes: Towards reagentless hydrogen peroxide sensing. Anal. Chim. Acta 2009, 648, 2–6. [Google Scholar] [CrossRef]
- Astuti, Y.; Topoglidis, E.; Durrant, J.R. Use of microperoxidase-11 to functionalize tin dioxide electrodes for the optical and electrochemical sensing of hydrogen peroxide. Anal. Chim. Acta 2011, 686, 126–132. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Li, P.; Xue, H.; Jia, N. A nanocomposite prepared from hemin and reduced graphene oxide foam for voltammetric sensing of hydrogen peroxide. Microchim. Acta 2019, 187, 45. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.M.; Seitz, W. Rudolf Chemiluminescence fiber optic probe for hydrogen peroxide based on the luminol reaction. Anal. Chem. 1978, 50, 1242–1246. [Google Scholar] [CrossRef]
- Olsson, B. Determination of hydrogen peroxide in a flow system with microperoxidase as catalyst for the luminol chemiluminescence reaction. Anal. Chim. Acta 1982, 136, 113–119. [Google Scholar] [CrossRef]
- Blum, L.J.; Plaza, J.M.; Coulet, P.R. Chemiluminescent Analyte Microdetection Based on the Luminol-H2 O 2 Reaction Using Peroxidase Immobilized on New Synthetic Membranes. Anal. Lett. 1987, 20, 317–326. [Google Scholar] [CrossRef]
- Hool, K.; Nieman, T.A. Immobilized luminol chemiluminescence reagent system for hydrogen peroxide determinations in flowing streams. Anal. Chem. 1988, 60, 834–837. [Google Scholar] [CrossRef]
- Navas Díaz, A.; Ramos Peinado, M.C.; Torijas Minguez, M.C. Sol–gel horseradish peroxidase biosensor for hydrogen peroxide detection by chemiluminescence. Anal. Chim. Acta 1998, 363, 221–227. [Google Scholar] [CrossRef]
- Ilyina, A.D.; Martínez Hernández, J.L.; López Luján, B.H.; Mauricio Benavides, J.E.; Romero García, J.; Rodríguez Martínez, J. Water quality monitoring using an enhanced chemiluminescent assay based on peroxidase-catalyzed peroxidation of luminol. Appl. Biochem. Biotechnol. 2000, 88, 45–58. [Google Scholar] [CrossRef]
- Ramos, M.C.; Torijas, M.C.; Díaz, A.N. Enhanced chemiluminescence biosensor for the determination of phenolic compounds and hydrogen peroxide. Sens. Actuators B Chem. 2001, 73, 71–75. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Jin, Y. Chemiluminescence flow biosensor for hydrogen peroxide with immobilized reagents. Sens. Actuators B Chem. 2001, 72, 115–119. [Google Scholar] [CrossRef]
- Yu, D.; Wang, P.; Zhao, Y.; Fan, A. Iodophenol blue-enhanced luminol chemiluminescence and its application to hydrogen peroxide and glucose detection. Talanta 2016, 146, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Yamashoji, S. Determination of viable mammalian cells by luminol chemiluminescence using microperoxidase. Anal. Biochem. 2009, 386, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Yu, X.; Zhao, J.; Sun, A.; Shi, X.; Li, D. An Electrochemiluminescence Sensor Based on Nafion/Magnetic Fe3O4 Nanocrystals Modified Electrode for the Determination of Bisphenol A in Environmental Water Samples. Sensors 2018, 18, 2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niazov, A.; Freeman, R.; Girsh, J.; Willner, I. Following Glucose Oxidase Activity by Chemiluminescence and Chemiluminescence Resonance Energy Transfer (CRET) Processes Involving Enzyme-DNAzyme Conjugates. Sensors 2011, 11, 10388–10397. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.-M.; Zhou, X.-L.; Wang, X.-N.; Li, P.; Xu, L.; Liu, E.-H. Miniaturized electrochemiluminescent biochip prepared on gold nanoparticles-loaded mesoporous silica film for visual detection of hydrogen peroxide released from living cells. Sens. Actuators B Chem. 2019, 284, 437–443. [Google Scholar] [CrossRef]
- Tian, H.; Tan, B.; Dang, X.; Zhao, H. Enhanced Electrochemiluminescence Detection for Hydrogen Peroxide Using Peroxidase-Mimetic Fe/N-Doped Porous Carbon. J. Electrochem. Soc. 2019, 166, B1594–B1601. [Google Scholar] [CrossRef]
- Yu, J.; Cao, M.; Wang, H.; Li, Y. Novel manganese (II)-based metal-organic gels: Synthesis, characterization and application to chemiluminescent sensing of hydrogen peroxide and glucose. Microchim. Acta 2019, 186, 696. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Feng, G.; Shan, H.; Huan, Y.; Fei, Q. Water-soluble Hemin-mPEG-enhanced Luminol Chemiluminescence for Sensitive Detection of Hydrogen Peroxide and Glucose. Anal. Sci. 2019, 35, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Marks, R.S.; Bassis, E.; Bychenko, A.; Levine, M.M. Chemiluminescent optical fiber immunosensor for detecting cholera antitoxin. OptEn 1997, 36, 3258–3264. [Google Scholar] [CrossRef]
- Huang, K.; Sun, Y.; Liu, L.; Hu, C. Chemiluminescence of 3-aminophthalic acid anion–hydrogen peroxide–cobalt (II). Luminescence 2020, 35, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Kawai, C.; Yamauchi, A.; Kuribayashi, F. A highly sensitive chemiluminescence assay for superoxide detection and chronic granulomatous disease diagnosis. Trop. Med. Health 2011, 39, 41–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamidate, T.; Katayama, A.; Ichihashi, H.; Watanabe, H. Characterization of peroxidases in luminol chemiluminescence coupled with copper-catalysed oxidation of cysteamine. J. Biolumin. Chemilumin. 1994, 9, 279–286. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Lin, W.-Y. Enhanced chemiluminescence for the oxidation of luminol with m-chloroperoxybenzoic acid catalyzed by microperoxidase 8. Anal. Bioanal. Chem. 2002, 372, 525–531. [Google Scholar] [CrossRef]
- Gorsuch, J.D.; Hercules, D.M. Studies on the chemiluminescence of luminol in dimethylsulfoxide and dimethylsulfoxide-water mixtures. Photochem. Photobiol. 1972, 15, 567–583. [Google Scholar] [CrossRef]
- Lee, J.; Seliger, H.H. Quantum Yields of the Luminol Chemiluminescence Reaction in Aqueous and Aprotic Solvents. Photochem. Photobiol. 1972, 15, 227–237. [Google Scholar] [CrossRef]
- Cormier, M.J.; Prichard, P.M. An Investigation of the Mechanism of the Luminescent Peroxidation of Luminol by Stopped Flow Techniques. J. Biol. Chem. 1968, 243, 4706–4714. [Google Scholar]
- Li, L.; Arnold, M.A.; Dordick, J.S. Mathematical model for the luminol chemiluminescence reaction catalyzed by peroxidase. Biotechnol. Bioeng. 1993, 41, 1112–1120. [Google Scholar] [CrossRef]
- Cercek, B.; Cercek, B.; Roby, K.; Cercek, L. Effect of oxygen abstraction on the peroxidase–luminol–perborate system: Relevance to the HRP enhanced chemiluminescence mechanism. J. Biolumin. Chemilumin. 1994, 9, 273–277. [Google Scholar] [CrossRef]
- Nakamura, M.; Nakamura, S. One- and Two-Electron Oxidations of Luminol by Peroxidase Systems. Free Radic. Biol. Med. 1998, 24, 537–544. [Google Scholar] [CrossRef]
- Navas Díaz, A.; González García, J.A. Nonlinear Multicomponent Kinetic Analysis for the Simultaneous Stopped-Flow Determination of Chemiluminescence Enhancers. Anal. Chem. 1994, 66, 988–993. [Google Scholar] [CrossRef]
- García Sanchez, F.; Navas Díaz, A.; González García, J.A. P-phenol derivatives as enhancers of the chemiluminescent luminol-horseradish peroxidase-H2O2 reaction: Substituent effects. J. Lumin. 1995, 65, 33–39. [Google Scholar] [CrossRef]
- Li, F.; Ma, W.; Liu, J.; Wu, X.; Wang, Y.; He, J. Luminol, horseradish peroxidase, and glucose oxidase ternary functionalized graphene oxide for ultrasensitive glucose sensing. Anal. Bioanal. Chem. 2018, 410, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jeschek, M.; Reuter, R.; Heinisch, T.; Trindler, C.; Klehr, J.; Panke, S.; Ward, T.R. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 2016, 537, 661–665. [Google Scholar] [CrossRef]
- Li, L.-L.; Yuan, H.; Liao, F.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. Rational design of artificial dye-decolorizing peroxidases using myoglobin by engineering Tyr/Trp in the heme center. Dalton Trans. 2017, 46, 11230–11238. [Google Scholar] [CrossRef]
- Yin, L.; Yuan, H.; Liu, C.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. A Rationally Designed Myoglobin Exhibits a Catalytic Dehalogenation Efficiency More than 1000-Fold That of a Native Dehaloperoxidase. ACS Catal. 2018, 9619–9624. [Google Scholar] [CrossRef]
- Hayashi, T.; Tinzl, M.; Mori, T.; Krengel, U.; Proppe, J.; Soetbeer, J.; Klose, D.; Jeschke, G.; Reiher, M.; Hilvert, D. Capture and characterization of a reactive haem–carbenoid complex in an artificial metalloenzyme. Nat. Catal. 2018, 1, 578–584. [Google Scholar] [CrossRef]
- Stenner, R.; Steventon, J.W.; Seddon, A.; Anderson, J.L.R. A de novo peroxidase is also a promiscuous yet stereoselective carbene transferase. Proc. Natl. Acad. Sci. USA 2020, 117, 1419–1428. [Google Scholar] [CrossRef] [Green Version]
- Chino, M.; Maglio, O.; Nastri, F.; Pavone, V.; DeGrado, W.F.; Lombardi, A. Artificial Diiron Enzymes with a De Novo Designed Four-Helix Bundle Structure. Eur. J. Inorg. Chem. 2015, 2015, 3371–3390. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, A.; Pirro, F.; Maglio, O.; Chino, M.; DeGrado, W.F. De Novo Design of Four-Helix Bundle Metalloproteins: One Scaffold, Diverse Reactivities. Acc. Chem. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chino, M.; Leone, L.; Zambrano, G.; Pirro, F.; D’Alonzo, D.; Firpo, V.; Aref, D.; Lista, L.; Maglio, O.; Nastri, F.; et al. Oxidation catalysis by iron and manganese porphyrins within enzyme-like cages. Biopolymers 2018, 109, e23107. [Google Scholar] [CrossRef] [PubMed]
- Perrella, F.; Raucci, U.; Chiariello, M.G.; Chino, M.; Maglio, O.; Lombardi, A.; Rega, N. Unveiling the structure of a novel artificial heme-enzyme with peroxidase-like activity: A theoretical investigation. Biopolymers 2018, 109, e23225. [Google Scholar] [CrossRef] [PubMed]
- Nastri, F.; D’Alonzo, D.; Leone, L.; Zambrano, G.; Pavone, V.; Lombardi, A. Engineering Metalloprotein Functions in Designed and Native Scaffolds. Trends Biochem. Sci. 2019. [Google Scholar] [CrossRef]
- Chino, M.; Leone, L.; Maglio, O.; D’Alonzo, D.; Pirro, F.; Pavone, V.; Nastri, F.; Lombardi, A. A De Novo Heterodimeric Due Ferri Protein Minimizes the Release of Reactive Intermediates in Dioxygen-Dependent Oxidation. Angew. Chem. Int. Ed. 2017, 56, 15580–15583. [Google Scholar] [CrossRef]
- Chino, M.; Leone, L.; Maglio, O.; Lombardi, A. Designing Covalently Linked Heterodimeric Four-Helix Bundles. Methods Enzymol. 2016, 580, 471–499. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Chino, M.; Liu, L.; Tang, Y.; Hu, X.; DeGrado, W.F.; Lombardi, A. De Novo Design of Tetranuclear Transition Metal Clusters Stabilized by Hydrogen-Bonded Networks in Helical Bundles. J. Am. Chem. Soc. 2018, 140, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Chino, M.; Zhang, S.-Q.; Pirro, F.; Leone, L.; Maglio, O.; Lombardi, A.; DeGrado, W.F. Spectroscopic and metal binding properties of a de novo metalloprotein binding a tetrazinc cluster. Biopolymers 2018, 109, e23339. [Google Scholar] [CrossRef]
- Nastri, F.; Lista, L.; Ringhieri, P.; Vitale, R.; Faiella, M.; Andreozzi, C.; Travascio, P.; Maglio, O.; Lombardi, A.; Pavone, V. A Heme-Peptide Metalloenzyme Mimetic with Natural Peroxidase-Like Activity. Chem. Eur. J. 2011, 17, 4444–4453. [Google Scholar] [CrossRef]
- Vitale, R.; Lista, L.; Cerrone, C.; Caserta, G.; Chino, M.; Maglio, O.; Nastri, F.; Pavone, V.; Lombardi, A. Artificial heme-enzyme with enhanced catalytic activity: Evolution, functional screening and structural characterization. Org. Biomol. Chem. 2015, 13, 4858–4868. [Google Scholar] [CrossRef]
- Zambrano, G.; Ruggiero, E.; Malafronte, A.; Chino, M.; Maglio, O.; Pavone, V.; Nastri, F.; Lombardi, A. Artificial Heme Enzymes for the Construction of Gold-Based Biomaterials. Int. J. Mol. Sci. 2018, 19, 2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caserta, G.; Chino, M.; Firpo, V.; Zambrano, G.; Leone, L.; D’Alonzo, D.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A. Enhancement of Peroxidase Activity in Artificial Mimochrome VI Catalysts through Rational Design. Chembiochem 2018, 19, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; D’Alonzo, D.; Balland, V.; Zambrano, G.; Chino, M.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A. Mn-Mimochrome VI*a: An Artificial Metalloenzyme with Peroxygenase Activity. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Firpo, V.; Le, J.M.; Pavone, V.; Lombardi, A.; Bren, K.L. Hydrogen evolution from water catalyzed by cobalt-mimochrome VI*a, a synthetic mini-protein. Chem. Sci. 2018, 9, 8582–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, J.M.; Alachouzos, G.; Chino, M.; Frontier, A.J.; Lombardi, A.; Bren, K.L. Tuning Mechanism through Buffer Dependence of Hydrogen Evolution Catalyzed by a Cobalt Mini-enzyme. Biochemistry 2020, 59, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, G.; Chino, M.; Renzi, E.; Di Girolamo, R.; Maglio, O.; Pavone, V.; Lombardi, A.; Nastri, F. Clickable artificial heme-peroxidases for the development of functional nanomaterials. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef]
- Pepich, B.V.; Dattilio, T.A.; Fair, P.S.; Munch, D.J.; Gordon, G.; Körtvélyesi, Z. An improved colorimetric method for chlorine dioxide and chlorite ion in drinking water using lissamine green B and horseradish peroxidase. Anal. Chim. Acta 2007, 596, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-W. Rational design of heme enzymes for biodegradation of pollutants toward a green future. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef]
- Carullo, P.; Chino, M.; Cetrangolo, G.P.; Terreri, S.; Lombardi, A.; Manco, G.; Cimmino, A.; Febbraio, F. Direct detection of organophosphate compounds in water by a fluorescence-based biosensing device. Sens. Actuators B Chem. 2018, 255, 3257–3266. [Google Scholar] [CrossRef]
- Cetrangolo, G.P.; Rusko, J.; Gori, C.; Carullo, P.; Manco, G.; Chino, M.; Febbraio, F. Highly Sensitive Detection of Chemically Modified Thio-Organophosphates by an Enzymatic Biosensing Device: An Automated Robotic Approach. Sensors 2020, 20, 1365. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambrano, G.; Nastri, F.; Pavone, V.; Lombardi, A.; Chino, M. Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence. Sensors 2020, 20, 3793. https://doi.org/10.3390/s20133793
Zambrano G, Nastri F, Pavone V, Lombardi A, Chino M. Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence. Sensors. 2020; 20(13):3793. https://doi.org/10.3390/s20133793
Chicago/Turabian StyleZambrano, Gerardo, Flavia Nastri, Vincenzo Pavone, Angela Lombardi, and Marco Chino. 2020. "Use of an Artificial Miniaturized Enzyme in Hydrogen Peroxide Detection by Chemiluminescence" Sensors 20, no. 13: 3793. https://doi.org/10.3390/s20133793