Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis
Abstract
:1. Introduction
2. Description, Architecture and Applications of CCSs
2.1. CCSs for Security
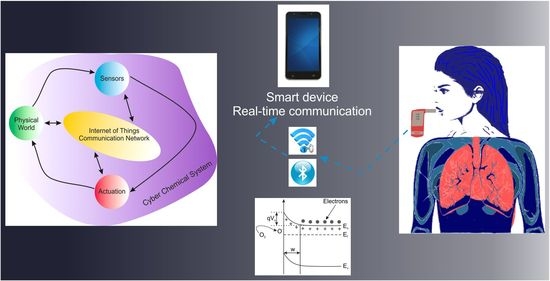
2.2. CCSs for Breath Analysis and Medical Diagnostics
3. Synthesis of Porous TiO2 Structures
3.1. ALD
3.2. Electrochemical Anodization
3.3. Hydrothermal Synthesis
4. Fundamentals and Design of Chemical Gas Sensors
5. Sensing Properties of TiO2
6. Conclusions and Outlook
Conflicts of Interest
References
- Morgan, J.; O’Donnell, G. Multi-sensor process analysis and performance characterisation in CNC turning—A cyber physical system approach. Int. J. Adv. Manuf. Technol. 2017, 92, 855–868. [Google Scholar] [CrossRef]
- Poudel, S.; Ni, Z.; Malla, N. Real-time cyber physical system testbed for power system security and control. Int. J. Electr. Power Energy Syst. 2017, 90, 124–133. [Google Scholar] [CrossRef]
- Lee, E.A. The past, present and future of cyber-physical systems: A focus on models. Sensors 2015, 15, 4837–4869. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.A.; Aziz, S.M.; Rahman, M. Review of cyber-physical system in healthcare. Int. J. Distrib. Sens. Netw. 2014, 10, 217415. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Parry, M.L. Potential impact of climate change on world food supply. Nature 1994, 367, 133–138. [Google Scholar] [CrossRef]
- Gou, Y.Y.; Hsu, Y.C.; Chao, H.R.; Que, D.E.; Tayo, L.L.; Lin, C.H.; Huang, S.M.; Tsai, C.H.; Shih, S.I. Pollution characteristics and diurnal variations in polybrominated diphenyl ethers in indoor and outdoor air from vehicle dismantler factories in southern taiwan. Aerosol Air Qual. Res. 2016, 16, 1931–1941. [Google Scholar] [CrossRef]
- Kiros, F.; Shakya, K.M.; Rupakheti, M.; Regmi, R.P.; Maharjan, R.; Byanju, R.M.; Naja, M.; Mahata, K.; Kathayat, B.; Peltier, R.E. Variability of anthropogenic gases: Nitrogen oxides, sulfur dioxide, ozone and ammonia in kathmandu valley, nepal. Aerosol Air Qual. Res. 2016, 16, 3088–3101. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Paulus, C.; Tabary, J.; Pierron, N.B.; Dinten, J.M.; Fabiani, E.; Mathy, F.; Mougel, F.; Rinkel, J.; Verger, L. A multi-energy x-ray backscatter system for explosives detection. J. Instrum. 2013, 8, P04003. [Google Scholar] [CrossRef]
- Forbes, T.P.; Sisco, E. Mass spectrometry detection and imaging of inorganic and organic explosive device signatures using desorption electro-flow focusing ionization. Anal. Chem. 2014, 86, 7788–7797. [Google Scholar] [CrossRef] [PubMed]
- Sinues, P.M.L.; Kohler, M.; Brown, S.A.; Zenobi, R.; Dallmann, R. Gauging circadian variation in ketamine metabolism by real-time breath analysis. Chem. Commun. 2017, 53, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Kholmanov, I.; Ponzoni, A.; Sberveglieri, V.; Poli, N.; Faglia, G.; Sberveglieri, G. A composite structure based on reduced graphene oxide and metal oxide nanomaterials for chemical sensors. Beilstein J. Nanotechnol. 2016, 7, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Tisch, U.; Haick, H. Chemical sensors for breath gas analysis: The latest developments at the breath analysis summit 2013. J. Breath Res. 2014, 8, 027103. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Seesaard, T.; Lorwongtragool, P.; Kerdcharoen, T. Development of fabric-based chemical gas sensors for use as wearable electronic noses. Sensors 2015, 15, 1885–1902. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Comini, E.; Ponzoni, A.; Sberveglieri, V.; Sberveglieri, G. ZnO quasi-1d nanostructures: Synthesis, modeling, and properties for applications in conductometric chemical sensors. Chemosensors 2016, 4, 6. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubes: Recent advances in synthesis and gas sensing properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Faglia, G.; Sberveglieri, G. Solid State Gas Sensing Preface; Springer: New York, NY, USA, 2009; pp. V–VI. [Google Scholar]
- Spencer, M.J.S. Gas sensing applications of 1d-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Ponzoni, A.; Ferroni, M.; Poli, N.; Bontempi, E.; Brisotto, M.; Faglia, G.; Sberveglieri, G. Large surface area biphase titania for chemical sensing. Sens. Actuators B Chem. 2015, 209, 1091–1096. [Google Scholar] [CrossRef]
- Comini, E.; Galstyan, V.; Faglia, G.; Bontempi, E.; Sberveglieri, G. Highly conductive titanium oxide nanotubes chemical sensors. Microporous Mesoporous Mater. 2015, 208, 165–170. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Vomiero, A.; Concina, I.; Braga, A.; Brisotto, M.; Bontempi, E.; Faglia, G.; Sberveglieri, G. Vertically aligned TiO2 nanotubes on plastic substrates for flexible solar cells. Small 2011, 7, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, M.G.; Passalacqua, R.; Abate, S.; Perathoner, S.; Centi, G.; Lanza, M.; Stassi, A. Functional nano-textured titania-coatings with self-cleaning and antireflective properties for photovoltaic surfaces. Sol. Energy 2016, 125, 227–242. [Google Scholar] [CrossRef]
- Chen, P.; Jin, Z.; Wang, Y.; Wang, M.; Chen, S.; Zhang, Y.; Wang, L.; Zhang, X.; Liu, Y. Interspace modification of titania-nanorod arrays for efficient mesoscopic perovskite solar cells. Appl. Surf. Sci. 2017, 402, 86–91. [Google Scholar] [CrossRef]
- Ellis, B.L.; Knauth, P.; Djenizian, T. Three-dimensional self-supported metal oxides for advanced energy storage. Adv. Mater. 2014, 26, 3368–3397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Aschauer, U.; Chen, J.; Selloni, A. Adsorption and reactions of O2 on anatase TiO2. Acc. Chem. Res. 2014, 47, 3361–3368. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–609. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. TrAC Trends Anal. Chem. 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Pereira, J.; Porto-Figueira, P.; Cavaco, C.; Taunk, K.; Rapole, S.; Dhakne, R.; Nagarajaram, H.; Câmara, S.J. Breath analysis as a potential and non-invasive frontier in disease diagnosis: An overview. Metabolites 2015, 5, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Yu, S.I.; Syu, H.Y. Development of the smart toilet equipment with measurements of physiological parameters. In Proceedings of the 2012 9th International Conference on Ubiquitous Intelligence & Computing and 9th International Conference on Autonomic & Trusted Computing (Uic/Atc), Fukuoka, Japan, 4–7 September 2012; pp. 9–16. [Google Scholar]
- Imai, H.; Takei, Y.; Shimizu, K.; Matsuda, M.; Hirashima, H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J. Mater. Chem. 1999, 9, 2971–2972. [Google Scholar] [CrossRef]
- Hoyer, P. Formation of a titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Song, J.; Zheng, M.; Yuan, X.; Li, Q.; Wang, F.; Ma, L.; You, Y.; Liu, S.; Liu, P.; Jiang, D.; et al. Electrochemically induced Ti3+ self-doping of TiO2 nanotube arrays for improved photoelectrochemical water splitting. J. Mater. Sci. 2017, 52, 6976–6986. [Google Scholar] [CrossRef]
- Terracciano, M.; Galstyan, V.; Rea, I.; Casalino, M.; De Stefano, L.; Sbervegleri, G. Chemical modification of TiO2 nanotube arrays for label-free optical biosensing applications. Appl. Surf. Sci. 2017, 419, 235–240. [Google Scholar] [CrossRef]
- Ghedini, E.; Nichele, V.; Signoretto, M.; Cerrato, G. Structure-directing agents for the synthesis of TiO2-based drug-delivery systems. Chem. Eur. J. 2012, 18, 10653–10660. [Google Scholar] [CrossRef] [PubMed]
- António, G.B.C.; Alexandre, C.B.; Vardan, G.; Guido, F.; Giorgio, S.; Isabel, M.M.S. Synthesis and electrochemical study of a hybrid structure based on PDMS-TEOS and titania nanotubes for biomedical applications. Nanotechnology 2014, 25, 365701. [Google Scholar]
- Park, J.Y.; Kim, H.H.; Rana, D.; Jamwal, D.; Katoch, A. Surface-area-controlled synthesis of porous TiO2 thin films for gas-sensing applications. Nanotechnology 2017, 28, 095502. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Hahn, R.; Cerri, I.; Schmuki, P. Fast growth of TiO2 nanotube arrays with controlled tube spacing based on a self-ordering process at two different scales. Electrochem. Commun. 2017, 77, 98–102. [Google Scholar] [CrossRef]
- Chang, Y.H.; Liu, C.M.; Chen, C.; Cheng, H.E.; Lu, T.C. The differences in optical characteristics of TiO2 and TiO2/AAO nanotube arrays fabricated by atomic layer deposition. J. Electrochem. Soc. 2012, 159, K136–K140. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wang, M.C.; He, H.T.; Li, H. Effect of heat treatment on the crystalline structure and hydrophilic properties of TiO2 porous thin films. J. Sol-Gel Sci. Technol. 2016, 80, 881–892. [Google Scholar] [CrossRef]
- Henrist, C.; Dewalque, J.; Cloots, R.; Vertruyen, B.; Jonlet, J.; Colson, P. Hierarchical porous TiO2 thin films by soft and dual templating a quantitative approach of specific surface and porosity. Thin Solid Films 2013, 539, 188–193. [Google Scholar] [CrossRef]
- Perez-Carrillo, L.A.; Vilchez, S.; Mosa, J.; Aparicio, M.; Castro, Y.; Duran, A.; Tascon, J.M.D.; Esquena, J. Synthesis and properties of TiO2-P2O5 and SiO2-TiO2-P2O5 porous hybrids obtained by templating in highly concentrated emulsions. Ceram. Int. 2016, 42, 18965–18973. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Q.; Mao, D.; Qi, J.; Wei, Y.Z.; Yu, R.B.; Li, A.R.; Li, S.Z.; Zhao, H.J.; Ma, Y.W.; et al. Dually ordered porous TiO2-rGO composites with controllable light absorption properties for efficient solar energy conversion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Mayon, Y.O.; Duong, T.; Nasiri, N.; White, T.P.; Tricoli, A.; Catchpole, K.R. Flame-made ultra-porous TiO2 layers for perovskite solar cells. Nanotechnology 2016, 27, 505403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Yu, Z.B.; Gao, Z.; Ge, H.B.; Zhao, S.C.; Chen, C.Q.; Chen, S.; Tong, X.L.; Wang, M.H.; Zheng, Z.F.; et al. Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2017, 56, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Borbon-Nunez, H.A.; Dominguez, D.; Munoz-Munoz, F.; Lopez, J.; Romo-Herrera, J.; Soto, G.; Tiznado, H. Fabrication of hollow TiO2 nanotubes through atomic layer deposition and mwcnt templates. Powder Technol. 2017, 308, 249–257. [Google Scholar] [CrossRef]
- Panda, S.K.; Shin, H. Electrochemical performance of amorphous and anatase TiO2 nanotube array-based anodes fabricated by atomic layer deposition. Mater. Res. Innov. 2015, 19, 694–699. [Google Scholar] [CrossRef]
- Chang, Y.H.; Liu, C.M.; Chen, C.; Cheng, H.E. The heterojunction effects of TiO2 nanotubes fabricated by atomic layer deposition on photocarrier transportation direction. Nanoscale Res. Lett. 2012, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Liu, C.M.; Chen, C.; Cheng, H.E. The effect of geometric structure on photoluminescence characteristics of 1-D TiO2 nanotubes and 2-D TiO2 films fabricated by atomic layer deposition. J. Electrochem. Soc. 2012, 159, D401–D405. [Google Scholar] [CrossRef]
- Huang, Y.J.; Pandraud, G.; Sarro, P.M. The atomic layer deposition array defined by etch-back technique: A new method to fabricate TiO2 nanopillars, nanotubes and nanochannel arrays. Nanotechnology 2012, 23, 485306. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, V.; Vomiero, A.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 nanotubular and nanoporous arrays by electrochemical anodization on different substrates. RSC Adv. 2011, 1, 1038–1044. [Google Scholar] [CrossRef]
- Xiao, S.S.; Bi, F.; Zhao, L.; Wang, L.Y.; Gai, G.Q. Design and synthesis of H-TiO2/MnO2 core-shell nanotube arrays with high capacitance and cycling stability for supercapacitors. J. Mater. Sci. 2017, 52, 7744–7753. [Google Scholar] [CrossRef]
- Jiang, J.; Fang, H.; Zhang, X.; He, K.; Wei, Z.; Pang, X.; Dai, J. Electrochemical synthesis of aligned amorphous carbon nanotubes/TiO2 nanotubes heterostructured arrays and its field emission properties. Diam. Relat. Mater. 2017, 74, 205–211. [Google Scholar] [CrossRef]
- Tang, X.H.; Raskin, J.P.; Lahem, D.; Krumpmann, A.; Decroly, A.; Debliquy, M. A formaldehyde sensor based on molecularly-imprinted polymer on a TiO2 nanotube array. Sensors 2017, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Baran, E.; Yazıcı, B. Preparation and characterization of poly (3-hexylthiophene) sensitized Ag doped TiO2 nanotubes and its carrier density under solar light illumination. Thin Solid Films 2017, 627, 82–93. [Google Scholar] [CrossRef]
- Ozkan, S.; Nguyen, N.T.; Hwang, I.; Mazare, A.; Schmuki, P. Highly conducting spaced TiO2 nanotubes enable defined conformal coating with nanocrystalline Nb2O5 and high performance supercapacitor applications. Small 2017, 13, 1603821. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liang, P.P.; Hu, Z.G.; Shi, L.Q.; Yang, X.; Sun, J.; Xu, N.; Wu, J.D. Enhanced photoelectrochemical activity of ZnO-coated TiO2 nanotubes and its dependence on ZnO coating thickness. Nanoscale Res. Lett. 2016, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.P.; Liao, X.M.; Sun, Y.; Yin, G.F.; Yao, Y.D.; Huang, Z.B.; Pu, X.M. Facile synthesis of a alpha-MoO3 nanoplate/TiO2 nanotube composite for high electrochemical performance. RSC Adv. 2017, 7, 22983–22989. [Google Scholar] [CrossRef]
- Han, C.; Shao, Q.; Liu, M.; Ge, S.; Liu, Q.; Lei, J. 5,10,15,20-tetrakis(4-chlorophenyl)porphyrin decorated TiO2 nanotube arrays: Composite photoelectrodes for visible photocurrent generation and simultaneous degradation of organic pollutant. Mater. Sci. Semicond. Process. 2016, 56, 166–173. [Google Scholar] [CrossRef]
- Lee, H.; Joo, H.-Y.; Yoon, C.; Lee, J.; Lee, H.; Choi, J.; Park, B.; Choi, T. Ferroelectric BiFeO3/TiO2 nanotube heterostructures for enhanced photoelectrochemical performance. Curr. Appl. Phys. 2017, 17, 679–683. [Google Scholar] [CrossRef]
- Ali, I.; Kim, S.-R.; Kim, S.-P.; Kim, J.-O. Anodization of bismuth doped TiO2 nanotubes composite for photocatalytic degradation of phenol in visible light. Catal. Today 2017, 282, 31–37. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Vomiero, A.; Borgese, L.; Bontempi, E.; Sberveglieri, G. Fabrication and investigation of gas sensing properties of Nb-doped TiO2 nanotubular arrays. Nanotechnology 2012, 23, 235706. [Google Scholar] [CrossRef] [PubMed]
- Chatzitakis, A.; Grandcolas, M.; Xu, K.; Mei, S.; Yang, J.; Jensen, I.J.T.; Simon, C.; Norby, T. Assessing the photoelectrochemical properties of C, N, F codoped TiO2 nanotubes of different lengths. Catal. Today 2017, 287, 161–168. [Google Scholar] [CrossRef]
- Sun, K.C.; Qadir, M.B.; Jeong, S.H. Hydrothermal synthesis of TiO2 nanotubes and their application as an over-layer for dye-sensitized solar cells. RSC Adv. 2014, 4, 23223–23230. [Google Scholar] [CrossRef]
- Aphairaj, D.; Wirunmongkol, T.; Niyomwas, S.; Pavasupree, S.; Limsuwan, P. Synthesis of anatase TiO2 nanotubes derived from a natural leucoxene mineral by the hydrothermal method. Ceram. Int. 2014, 40, 9241–9247. [Google Scholar] [CrossRef]
- Jo, W.K.; Lee, J.Y.; Chun, H.H. Titania nanotubes grown on carbon fibers for photocatalytic decomposition of gas-phase aromatic pollutants. Materials 2014, 7, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, S.H.; Shi, J.W.; Niu, C.M. Highly crystallized C-doped mesoporous anatase TiO2 with visible light photocatalytic activity. Catalysts 2016, 6, 117. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Q.; Li, X.; Chen, X. Characterization and photocatalytic activity of large-area single crystalline anatase TiO2 nanotube films hydrothermal synthesized on plasma electrolytic oxidation seed layers. J. Alloys Compd. 2014, 597, 101–109. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Ryu, J.; Lim, J.M.; Oh, B.T.; Park, J.H.; Kim, B.G.; Jang, J.S. Hydrothermal synthesis of titanate nanotubes from TiO2 nanorods prepared via a molten salt flux method as an effective adsorbent for strontium ion recovery. RSC Adv. 2016, 6, 98449–98456. [Google Scholar] [CrossRef]
- Chen, Q.F.; Sun, X.N.; Liu, A.P.; Zhang, Q.F.; Cao, G.Z.; Zhou, X.Y. Photovoltaic performance of dye-sensitized solar cells using TiO2 nanotubes aggregates produced by hydrothermal synthesis. Int. J. Mod. Phys. B 2015, 29, 1542050. [Google Scholar] [CrossRef]
- Cho, S.H.; Adhikari, R.; Kim, S.H.; Kim, T.H.; Lee, S.W. Microwave assisted hydrothermal synthesis and structural characterization of TiO2 nanotubes. J. Nanosci. Nanotechnol. 2015, 15, 7391–7394. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, A.; Muthukumarasamy, N.; Thambidurai, M.; Velauthapillai, D.; Agilan, S.; Balasundaraprabhu, R. Effect of reaction time on the formation of TiO2 nanotubes prepared by hydrothermal method. Optik 2015, 126, 2491–2494. [Google Scholar] [CrossRef]
- Chu, M.; Tang, Y.; Rong, N.; Cui, X.; Liu, F.; Li, Y.; Zhang, C.; Xiao, P.; Zhang, Y. Hydrothermal synthesis, and tailoring the growth of Ti-supported TiO2 nanotubes with thick tube walls. Mater. Des. 2016, 97, 257–267. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, M.; Zhu, G.; Li, D.S.; Yan, D.; Lu, T.; Pan, L.K. Porous cake-like TiO2 derived from metal-organic frameworks as superior anode material for sodium ion batteries. Ceram. Int. 2017, 43, 2398–2402. [Google Scholar] [CrossRef]
- Razali, M.H.; Noor, A.F.M.; Yusoff, M. Hydrothermal synthesis and characterization of Cu2+/F− co-doped titanium dioxide (TiO2) nanotubes as photocatalyst for methyl orange degradation. Sci. Adv. Mater. 2017, 9, 1032–1041. [Google Scholar] [CrossRef]
- Zhang, P.; Mo, Z.; Wang, Y.; Han, L.; Zhang, C.; Zhao, G.; Li, Z. One-step hydrothermal synthesis of magnetic responsive TiO2 nanotubes/Fe3O4/graphene composites with desirable photocatalytic properties and reusability. RSC Adv. 2016, 6, 39348–39355. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Cui, X.; Yao, W.; Duan, T. One-step hydrothermal synthesis of iron and nitrogen co-doped TiO2 nanotubes with enhanced visible-light photocatalytic activity. CrystEngComm 2015, 17, 8368–8376. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.Z.; Wang, C.M.; Zhang, G.S.; Song, Q.; Sun, W.X.; Song, X.J.; Sun, M.Y.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349. [Google Scholar] [CrossRef]
- Reddy, N.L.; Kumar, S.; Krishnan, V.; Sathish, M.; Shankar, M.V. Multifunctional Cu/Ag quantum dots on TiO2 nanotubes as highly efficient photocatalysts for enhanced solar hydrogen evolution. J. Catal. 2017, 350, 226–239. [Google Scholar] [CrossRef]
- Niu, X.; Yu, J.; Wang, L.; Fu, C.; Wang, J.; Wang, L.; Zhao, H.; Yang, J. Enhanced photocatalytic performance of TiO2 nanotube based heterojunction photocatalyst via the coupling of graphene and FTO. Appl. Surf. Sci. 2017, 413, 7–15. [Google Scholar] [CrossRef]
- Wei, X.Z.; Wang, H.J.; Zhu, G.F.; Chen, J.Y.; Zhu, L.P. Iron-doped TiO2 nanotubes with high photocatalytic activity under visible light synthesized by an ultrasonic-assisted sol-hydrothermal method. Ceram. Int. 2013, 39, 4009–4016. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B Chem. 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Sakai, G.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuators B Chem. 2001, 80, 125–131. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Academic press: Boston, MA, USA, 1989; pp. 1–556. [Google Scholar]
- Costanzo, F.; Silvestrelli, P.L.; Ancilotto, F. Physisorption, diffusion, and chemisorption pathways of H2 molecule on graphene and on (2,2) carbon nanotube by first principles calculations. J. Chem. Theory Comput. 2012, 8, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zakrzewska, K. Gas sensing mechanism of TiO2-based thin films. Vacuum 2004, 74, 335–338. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Radecka, M. TiO2-based nanomaterials for gas sensing-influence of anatase and rutile contributions. Nanoscale Res. Lett. 2017, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Rebholz, J.; Weimar, U. Conduction mechanism switch for SnO2 based sensors during operation in application relevant conditions; implications for modeling of sensing. Sens. Actuators B Chem. 2015, 207, 455–459. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Lu, M.; Zuo, L. A novel polyimide based micro heater with high temperature uniformity. Sens. Actuators A Phys. 2017, 257, 58–64. [Google Scholar] [CrossRef]
- Quintero, A.V.; Molina-Lopez, F.; Smits, E.C.P.; Danesh, E.; van den Brand, J.; Persaud, K.; Oprea, A.; Barsan, N.; Weimar, U.; de Rooij, N.F.; et al. Smart rfid label with a printed multisensor platform for environmental monitoring. Flex. Print. Electron. 2016, 1. [Google Scholar] [CrossRef]
- Rieu, M.; Camara, M.; Tournier, G.; Viricelle, J.P.; Pijolat, C.; de Rooij, N.F.; Briand, D. Fully inkjet printed SnO2 gas sensor on plastic substrate. Sens. Actuators B Chem. 2016, 236, 1091–1097. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Ponzoni, A.; Bontempi, E.; Brisotto, M.; Faglia, G.; Sberveglieri, G. Synthesis of self-assembled chain-like zno nanostructures on stiff and flexible substrates. Crystengcomm 2013, 15, 2881–2887. [Google Scholar] [CrossRef]
- Jaaniso, R.; Tan, O.K. Semiconductor Gas Sensors; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 1–552. [Google Scholar]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.S.; Wang, Z.L.; Xie, Z.X.; Zheng, L.S. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed]
- Parthibavarman, M.; Hariharan, V.; Sekar, C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Tso, C.P.; Zhung, C.M.; Shih, Y.H.; Tseng, Y.M.; Wu, S.C.; Doong, R.A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sadaoka, Y.; Mori, M. Detection of VOC in air with a planar-type potentiometric gas sensor based on YSZ with a Pt electrode modified with TiO2. Sens. Actuators B Chem. 2017, 248, 878–885. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Sun, L.; Fang, W.; Yang, Y.; Yu, H.; Wang, T.; Dong, X.; Liu, G.; Wang, J.; Yu, W.; Shi, K. Highly active and porous single-crystal In2O3 nanosheet for nox gas sensor with excellent response at room temperature. RSC Adv. 2017, 7, 33419–33425. [Google Scholar] [CrossRef]
- Mirzaei, A.; Sun, G.-J.; Lee, J.K.; Lee, C.; Choi, S.; Kim, H.W. Hydrogen sensing properties and mechanism of NiO-Nb2O5 composite nanoparticle-based electrical gas sensors. Ceram. Int. 2017, 43, 5247–5254. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Fu, X.; Zhang, X.; Meng, F.; Yu, X.; Jin, Z.; Kong, L.; Liu, J. Modification of coral-like SnO2 nanostructures with dense TiO2 nanoparticles for a self-cleaning gas sensor. Talanta 2012, 99, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, G.; Wang, D.; Liu, C.; Zhang, Z. A self-cleaning coating material of TiO2 porous microspheres/cement composite with high-efficient photocatalytic depollution performance. Mater. Lett. 2017, 200, 1–5. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, G.; Zhao, Z.; Tan, H.; Yu, W.; Zhang, X.; Sun, Z. TiO2 nanosheet array thin film for self-cleaning coating. RSC Adv. 2015, 5, 9861–9864. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, Y.; Gong, J.; Gong, D.; Chi, L.; Lin, C.; Chen, Z. Transparent superhydrophobic/superhydrophilic TiO2-based coatings for self-cleaning and anti-fogging. J. Mater. Chem. 2012, 22, 7420–7426. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Burgess, K.; Wang, L.; Chowdhury, R.R.; Lotus, A.F.; Moula, G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology 2012, 23, 425606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Yu, L.; Tie, J.; Dong, X.C. Gas sensitivity and sensing mechanism studies on Au-doped TiO2 nanotube arrays for detecting SF6 decomposed components. Sensors 2014, 14, 19517–19532. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.; Park, S.; Lee, W.I.; Lee, C. Effects of functionalization of TiO2 nanotube array sensors with pd nanoparticles on their selectivity. Sensors 2014, 14, 15849–15860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tie, J.; Zhang, J.B. A Pt-doped TiO2 nanotube arrays sensor for detecting SF6 decomposition products. Sensors 2013, 13, 14764–14776. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Hedman, H.-P.; Kemell, M.; Tuominen, A.; Punkkinen, R. Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B Chem. 2016, 222, 190–197. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsueh, Y.C.; Lee, P.S.; Wang, C.C.; Wu, J.M.; Perng, T.P.; Shih, H.C. Fabrication of tin dioxide nanowires with ultrahigh gas sensitivity by atomic layer deposition of platinum. J. Mater. Chem. 2011, 21, 10552–10558. [Google Scholar] [CrossRef]
- Hübner, M.; Bârsan, N.; Weimar, U. Influences of Al, Pd and Pt additives on the conduction mechanism as well as the surface and bulk properties of SnO2 based polycrystalline thick film gas sensors. Sens. Actuators B Chem. 2012, 171, 172–180. [Google Scholar] [CrossRef]
- Hübner, M.; Hafner, S.; Bârsan, N.; Weimar, U. The influence of Pt doping on the sensing and conduction mechanism of SnO2 based thick film sensors. Procedia Eng. 2011, 25, 104–107. [Google Scholar] [CrossRef]
- Li, Z.H.; Ding, D.Y.; Liu, Q.; Ning, C.Q.; Wang, X.W. Ni-doped TiO2 nanotubes for wide-range hydrogen sensing. Nanoscale Res. Lett. 2014, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, D.; Liu, Q.; Ning, C. Hydrogen sensing with Ni-doped TiO2 nanotubes. Sensors 2013, 13, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sens. Actuators B Chem. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Sennik, E.; Kilinc, N.; Ozturk, Z.Z. Electrical and VOC sensing properties of anatase and rutile TiO2 nanotubes. J. Alloys Compd. 2014, 616, 89–96. [Google Scholar] [CrossRef]
- Kılınç, N.; Şennik, E.; Işık, M.; Ahsen, A.Ş.; Öztürk, O.; Öztürk, Z.Z. Fabrication and gas sensing properties of C-doped and un-doped TiO2 nanotubes. Ceram. Int. 2014, 40, 109–115. [Google Scholar] [CrossRef]
- Li, Z.H.; Ding, D.Y.; Ning, C.Q. P-type hydrogen sensing with Al- and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-dimensional MoS2-decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloys Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective voc sensors. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.X.; Zou, Y.C.; Li, G.D.; Wang, P.P.; Chen, J.S. Porous titania with heavily self-doped Ti3+ for specific sensing of CO at room temperature. Inorg. Chem. 2013, 52, 5924–5930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, Y.Z.; Huang, S.S.; Ren, S.T.; Li, P.; Li, J.J. Resistive and capacitive response of nitrogen-doped TiO2 nanotubes film humidity sensor. Nanotechnology 2011, 22, 025501. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.I.; Linder, R.; Perriello, G.; Di Daniele, N.; Poppl, S.J.; De Lorenzo, A. Predicting type 2 diabetes using an electronic nose-base artificial neural network analysis. Diabetes Nutr. Metab. 2002, 15, 215–221. [Google Scholar] [PubMed]
- Teshima, N.; Li, J.; Toda, K.; Dasgupta, P.K. Determination of acetone in breath. Anal. Chim. Acta 2005, 535, 189–199. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Leonardi, S.G.; Pullar, R.C.; Seabra, M.P.; Neri, G.; Labrincha, J.A. Sensing properties and photochromism of Ag-TiO2 nano-heterostructures. J. Mater. Chem. A 2016, 4, 9600–9613. [Google Scholar] [CrossRef]














| Pore Width (nm) | Type of Pore |
|---|---|
| ≤2 | Micropores |
| 2–50 | Mesopores |
| >50 | Macropores |
| Shape/Composition | TiO2 Crystalline Structure | Synthesis Method | Operating Temperature (°C) | Target Gas, Concentration | Response | Response/Recovery Times | Ref. |
|---|---|---|---|---|---|---|---|
| Tubular Au-TiO2 | Anatase | Anodization | 110 | SO2F2, 50 ppm | (ΔR/R0)·100%, 19.95% | - | [114] |
| Tubular Pd-TiO2 | Anatase | Anodization | 200 | Ethanol, 10–3000 ppm | (ΔR/R0)·100%, 297–21,253% | 10.2/7.1 s | [115] |
| Tubular Pt-TiO2 | Anatase | Anodization | 150 | SO2F2, 30–100 ppm | (ΔR/R0)·100%, ~8.65–38% | - | [116] |
| Tubular Ni-TiO2 | Anatase | Anodization | 200 | H2, 1000 ppm | (ΔR/R0)·100%, 40% | - | [121] |
| Tubular Ni-TiO2 | Anatase | Anodization | 200 | H2, 1000 ppm | (ΔR/R0)·100%, 13.7% | 80/- s | [122] |
| Tubular Cr-TiO2 | Anatase | Anodization, soaking, thermal treatment | 500 | NO2, 10–100 ppm | ΔR/R0, ~2–3.5 | -/8–24 min | [123] |
| Tubular Nb-TiO2 | Anatase, rutile | Anodization | 400 | Ethanol, 50 ppm | ΔG/G0, ~6 | 120/120 s | [20] |
| Tubular Nb-TiO2 | Anatase | Anodization | 300 | Acetone, 25 ppm | ΔG/G0,~7 | - | [65] |
| Tubular TiO2 | Anatase | Anodization | 200 | Ethanol, 5000 ppm | (ΔG/G0)·100%, ~300% | - | [124] |
| Tubular C-TiO2 | Anatase | Anodization, thermal treatment | 100 | H2, 5000 ppm | ΔG/G0, ~2 | - | [125] |
| Tubular Al-V-TiO2 | Anatase | Anodization | 300 | H2, 1000 ppm | (ΔR/R0)·100%, 50% | - | [126] |
| Tubular MoS2-TiO2 | Anatase | Anodization, hydrothermal growth | 150 | Ethanol, 100 ppm | R/R0, 14.2 | - | [127] |
| Porous Ag-SnO2-TiO2 | Anatase TiO2 | Chemical approaches, thermal treatment | 275 | Ethanol, 50 ppm | R0/R, ~53 | 3.5/7 s | [128] |
| Porous Ti3+-TiO2 | Anatase, rutile | Chemical approaches, thermal treatment | Room temperature | CO, 100 ppm | R0/R, ~1.6 | - | [129] |
| Tubular, polypyrrole based polymer-TiO2 | - | Anodization, electropolymerization | Room temperature | CH2O, 1 ppm | ΔG/G0, 13% | - | [57] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galstyan, V. Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis. Sensors 2017, 17, 2947. https://doi.org/10.3390/s17122947
Galstyan V. Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis. Sensors. 2017; 17(12):2947. https://doi.org/10.3390/s17122947
Chicago/Turabian StyleGalstyan, Vardan. 2017. "Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis" Sensors 17, no. 12: 2947. https://doi.org/10.3390/s17122947