Multi-Proxy Paleoecological Reconstruction of Peatland Initiation, Development and Restoration in an Urban Area (Moscow, Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Study Site
2.3. Peat Coring and Sampling
2.4. Chronology of the Peat Deposits
2.5. Loss on Ignition (LOI) and Peat Humification
2.6. Plant Macrofossil Analysis
2.7. Pollen Analysis
2.8. Testate Amoeba Analysis
2.9. Macrocharcoal Analysis
2.10. Data Analyses
3. Results
3.1. Peat Stratigraphy, Chronology and Properties
3.2. Plant Macrofossils
3.3. Pollen Analysis
3.4. Testate Amoeba Analysis
3.5. Macrocharcoal Analysis
4. Discussion
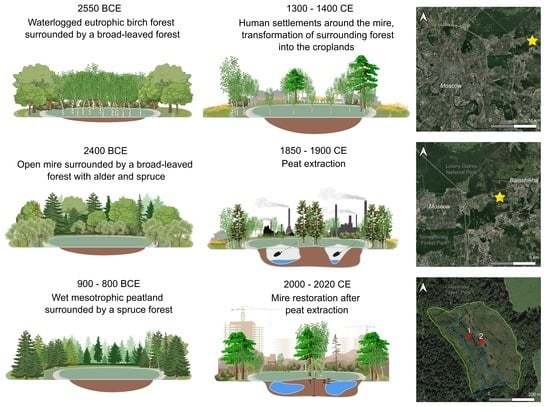
4.1. Peatland Initiation
4.2. Development of the Peatland and the Surrounding Landscape
4.3. Peatland Restoration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montanarella, L.; Jones, R.J.A.; Hiederer, R. The Distribution of Peatland in Europe. Mires Peat 2006, 1, 1. [Google Scholar]
- Rydin, H.; Jeglum, J.K.; Bennett, K.D. The Biology of Peatlands, 2nd ed.; OUP: Oxford, UK, 2013; ISBN 978-0-19-960299-5. [Google Scholar]
- Gorham, E. Northern Peatlands: Role in the Carbon Cycle and Probable Responses to Climatic Warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Blodau, C. Carbon Cycling in Peatlands: A Review of Processes and Controls. Environ. Rev. 2002, 10, 111–134. [Google Scholar] [CrossRef]
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.-S.; Roulet, N. Peatlands in the Earth’s 21st Century Climate System. Environ. Rev. 2011, 19, 371–396. [Google Scholar] [CrossRef]
- Holden, J. Peatland Hydrology and Carbon Release: Why Small-Scale Process Matters. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2005, 363, 2891–2913. [Google Scholar] [CrossRef] [Green Version]
- Janse, J.H.; van Dam, A.A.; Hes, E.M.A.; de Klein, J.J.M.; Finlayson, C.M.; Janssen, A.B.G.; van Wijk, D.; Mooij, W.M.; Verhoeven, J.T.A. Towards a Global Model for Wetlands Ecosystem Services. Curr. Opin. Environ. Sustain. 2019, 36, 11–19. [Google Scholar] [CrossRef]
- Prentice, C. Records of Vegetation in Time and Space: The Principles of Pollen Analysis. In Vegetation History; Handbook of Vegetation Science; Huntley, B., Webb, T., Eds.; Springer: Dordrecht, Netherlands, 1988; pp. 17–42. ISBN 978-94-009-3081-0. [Google Scholar]
- Ponomarenko, E.V.; Ershova, E.G.; Krenke, N.A.; Bakumenko, V.O. Traces of Iron Age slash-and-burn agriculture under the Slavic kurgans at the MSU Zvenigorod Biological Station. Brief Commun. Inst. Archaeol. 2021, 263, 60–73. [Google Scholar] [CrossRef]
- Whitlock, C.; Bartlein, P.J. Holocene Fire Activity as a Record of Past Environmental Change. In Developments in Quaternary Sciences; The Quaternary Period in the United States; Elsevier: Amsterdam, Netherlands, 2003; Volume 1, pp. 479–490. [Google Scholar]
- Higuera, P.E.; Peters, M.E.; Brubaker, L.B.; Gavin, D.G. Understanding the Origin and Analysis of Sediment-Charcoal Records with a Simulation Model. Quat. Sci. Rev. 2007, 26, 1790–1809. [Google Scholar] [CrossRef]
- Whitlock, C.; Higuera, P.E.; McWethy, D.B.; Briles, C.E. Paleoecological Perspectives on Fire Ecology: Revisiting the Fire-Regime Concept. Open Ecol. J. 2010, 3, 6–23. [Google Scholar] [CrossRef] [Green Version]
- Vannière, B.; Colombaroli, D.; Chapron, E.; Leroux, A.; Tinner, W.; Magny, M. Climate versus Human-Driven Fire Regimes in Mediterranean Landscapes: The Holocene Record of Lago Dell’Accesa (Tuscany, Italy). Quat. Sci. Rev. 2008, 27, 1181–1196. [Google Scholar] [CrossRef] [Green Version]
- Birks, H.H. Plant Macrofossil Introduction. Encycl. Quat. Sci. 2007, 3, 2266–2288. [Google Scholar]
- Mauquoy, D.; Hughes, P.; Van Geel, B. A Protocol for Plant Macrofossil Analysis of Peat Deposits. Mires Peat 2010, 7, 6. [Google Scholar]
- Hughes, P.D.M.; Barber, K.E. Contrasting Pathways to Ombrotrophy in Three Raised Bogs from Ireland and Cumbria, England. Holocene 2004, 14, 65–77. [Google Scholar] [CrossRef]
- Charman, D.J. Biostratigraphic and Palaeoenvironmental Applications of Testate Amoebae. Quat. Sci. Rev. 2001, 20, 1753–1764. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Charman, D.J.; Warner, B.G. Testate Amoebae Analysis in Ecological and Paleoecological Studies of Wetlands: Past, Present and Future. Biodivers. Conserv. 2008, 17, 2115–2137. [Google Scholar] [CrossRef] [Green Version]
- Chambers, F.M.; Beilman, D.W.; Yu, Z. Methods for Determining Peat Humification and for Quantifying Peat Bulk Density, Organic Matter and Carbon Content for Palaeostudies of Climate and Peatland Carbon Dynamics. Mires Peat 2010, 7, 7. [Google Scholar]
- Novenko, E.Y.; Tsyganov, A.N.; Volkova, E.M.; Kupriyanov, D.A.; Mironenko, I.V.; Babeshko, K.V.; Utkina, A.S.; Viktor, P.; Mazei, Y.A. Mid- and Late Holocene Vegetation Dynamics and Fire History in the Boreal Forest of European Russia: A Case Study from Meshchera Lowlands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 459, 570–584. [Google Scholar] [CrossRef]
- Rudenko, O.V.; Volkova, E.M.; Babeshko, K.V.; Tsyganov, A.N.; Mazei, Y.A.; Novenko, E.Y. Late Holocene Vegetation Dynamics and Human Impact in the Catchment Basin of the Upper Oka River (Mid-Russian Uplands): A Case Study from the Orlovskoye Polesye National Park. Quat. Int. 2019, 504, 118–127. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Zyuganova, I.S.; Volkova, E.M.; Dyuzhova, K.V. A 7000-Year Pollen and Plant Macrofossil Record from the Mid-Russian Upland, European Russia: Vegetation History and Human Impact. Quat. Int. 2019, 504, 70–79. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Eremeeva, A.P.; Chepurnaya, A.A. Reconstruction of Holocene Vegetation, Tree Cover Dynamics and Human Disturbances in Central European Russia, Using Pollen and Satellite Data Sets. Veg. Hist. Archaeobotany 2014, 23, 109–119. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Volkova, E.; Glasko, M.; Zuganova, I. Palaeoecological Evidence for the Middle and Late Holocene Vegetation, Climate and Land Use in the Upper Don River Basin (Russia). Veg. Hist. Archaeobotany 2012, 21, 337–352. [Google Scholar] [CrossRef]
- Payne, R.J.; Malysheva, E.; Tsyganov, A.N.; Pampura, T.; Novenko, E.Y.; Volkova, E.M.; Babeshko, K.V.; Mazei, Y.A. A Multi-Proxy Record of Holocene Environmental Change, Peatland Development and Carbon Accumulation from Staroselsky Moch Peatland, Russia. Holocene 2016, 26, 314–326. [Google Scholar] [CrossRef]
- Nosova, M.; Severova, E.; Volkova, O. A 6500-Year Pollen Record from the Polistovo-Lovatskaya Mire System (Northwest European Russia). Vegetation Dynamics and Signs of Human Impact. Grana 2017, 56, 410–423. [Google Scholar] [CrossRef]
- Tsyganov, A.N.; Kupriyanov, D.A.; Babeshko, K.V.; Borisova, T.V.; Chernyshov, V.A.; Volkova, E.M.; Chekova, D.A.; Mazei, Y.A.; Novenko, E.Y. Autogenic and Allogenic Factors Affecting Development of a Floating Sphagnum-Dominated Peat Mat in a Karst Pond Basin. Holocene 2019, 29, 120–129. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Tsyganov, A.N.; Bobrovsky, M.V.; Mazei, N.G.; Kupriyanov, D.A.; Gałka, M.; Rostanets, D.V.; Khazanova, K.P.; Stoiko, T.G.; Pastukhova, Y.A.; et al. Peatland Development, Vegetation History, Climate Change and Human Activity in Valdai Uplands (Central European Russia) during the Holocene: A Multi-Proxy Palaeoecological Study. Diversity 2020, 12, 462. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Kusilman, M.V.; Olchev, A.V. Peatland Initiation in Central European Russia during the Holocene: Effect of Climate Conditions and Fires. Holocene 2021, 31, 545–555. [Google Scholar] [CrossRef]
- Kupriyanov, D.A.; Novenko, E.Y. Reconstruction of the Holocene Dynamics of Forest Fires in the Central Part of Meshcherskaya Lowlands According to Antracological Analysis. Contemp. Probl. Ecol. 2019, 12, 204–212. [Google Scholar] [CrossRef]
- Sirin, A.; Minayeva, T.; Yurkovskaya, T.; Kuznetsov, O.; Smagin, V.; Fedotov, Y. Russian Federation (European Part). In Mires and Peatlands of Europe: Status, Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017; p. 780. [Google Scholar]
- Tcvetkov, P. The History, Present Status and Future Prospects of the Russian Fuel Peat Industry. Mires Peat 2017, 19, 14. [Google Scholar] [CrossRef]
- Wheeler, B.D.; Shaw, S.C.; Fojt, W.J.; Robertson, R.A. Restoration of Temperate Wetlands, 1st ed.; Wiley: Hoboken, NJ, USA, 1995; ISBN 978-0-471-95105-6. [Google Scholar]
- Andersen, R.; Farrell, C.; Graf, M.; Muller, F.; Calvar, E.; Frankard, P.; Caporn, S.; Anderson, P. An Overview of the Progress and Challenges of Peatland Restoration in Western Europe. Restor. Ecol. 2017, 25, 271–282. [Google Scholar] [CrossRef]
- Gorham, E.; Rochefort, L. Peatland Restoration: A Brief Assessment with Special Reference to Sphagnum Bogs. Wetl. Ecol. Manag. 2003, 11, 109–119. [Google Scholar] [CrossRef]
- Chimner, R.A.; Cooper, D.J.; Wurster, F.C.; Rochefort, L. An Overview of Peatland Restoration in North America: Where Are We after 25 Years? Restor. Ecol. 2017, 25, 283–292. [Google Scholar] [CrossRef]
- Aleksandrova, V.D.; Yurkovskaya, T.K. Geobotanical Zoning of the Non-Chernozem Region of the European Part of the RSFSR; Nauka: St. Petersburg, Russia, 1989; ISBN 5-02-026546-2. (In Russian) [Google Scholar]
- Krenke, A.N. Antiquities of the Moskva River Basin from the Neolithic to the Middle Ages: Stages of Cultural Development, the Formation of a Productive Economy and An Anthropogenic Landscape; Svitok: Smolensk, Russia, 2019; 392p. (In Russian) [Google Scholar]
- Chernov, S.Z. Rural settlements and landscapes on Pekhorka: The riddle of economic recovery in Meshchora under the first Moscow Princes. In Culture of Medieval Moscow. Historical Landscapes; Nauka: Moscow, Russia, 2005; Volume 1, pp. 126–188. (In Russian) [Google Scholar]
- Chernov, S.Z. Domain of Moscow dukes in urban camps. 1271–1505 years (Acts of Moscow Rus. Micro-regional studies. Volume 2). In Culture of Medieval Moscow. Historical Landscapes; Nauka: Moscow, Russia, 2005; Volume 2, pp. 51–55. [Google Scholar]
- Galanin, A.E. Balashikha in Persons and Biographies: Encyclopedic Dictionary (to the 175th Anniversary of Balashikha); Delta: Moscow, Russia, 2005; ISBN 5-902370-29-9. (In Russian) [Google Scholar]
- Vleeschouwer, F.; Chambers, F.; Swindles, G. Coring and Sub-Sampling of Peatlands for Palaeoenvironmental Research. Mires Peat 2010, 7, 1. [Google Scholar]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 Cal KBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.A.; Vazquez, J.E.; Goring, S. clam: Classical Age-Depth Modelling of Cores from Deposits 2022. R Package Version 2.5.0. Available online: https://CRAN.R-project.org/package=clam (accessed on 27 December 2022).
- Blaauw, M.; Christen, J.A. Flexible Paleoclimate Age-Depth Models Using an Autoregressive Gamma Process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, J.; Aquino-Lopez, M. rplum: Bayesian Age-Depth Modelling of Cores Dated by Pb-210, R Package Version 0.2.2, 2021. Available online: https://cran.r-project.org/package=rplum (accessed on 27 December 2022).
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on Ignition as a Method for Estimating Organic and Carbonate Content in Sediments: Reproducibility and Comparability of Results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Katz, N.Y.; Katz, S.V.; Skobeeva, E.I. Atlas of Plant Residues in Peats; UDK: 553.97.06 (084.4); Nedra: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Ignatov, M.S.; Ignatova, E.A. Flora of Mosses in the Middle part of European Russia. T. 1: Sphagnaceae-Hedwiigiaceae; KMK: Moscow, Russia, 2003; Volume 1, ISBN 5-87317-104-1. (In Russian) [Google Scholar]
- Laine, J.; Harju, P.; Timonen, T.; Laine, A.; Tuittila, E.-S.; Minkkinen, K.; Vasander, H. The Intricate Beauty of Sphagnum Mosses-A Finnish Guide to Identification; University of Helsinki, Department of Forest Ecology: Helsinki, Finland, 2009; ISBN 978-952-10-5617-8. [Google Scholar]
- Gajewski, K. Preparation of Organic Sediments for Pollen Analysis. Available online: http://www.lpc.uottawa.ca/resources/pollen.html (accessed on 23 November 2009).
- Moore, P.D.; Webb, J.A.; Collison, M.E. Pollen Analysis, 2nd ed.; Blackwell: Oxford, UK, 1991; ISBN 9780865428959. [Google Scholar]
- Faegri, K.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis, 4th ed.; John Wiley & Sons Ltd.: Chichester, UK, 1989; ISBN 0-471-92178-5. [Google Scholar]
- Mazei, Y.A.; Chernyshov, V.A. Testate Amoebae Communities in the Southern Tundra and Forest-Tundra of Western Siberia. Biol. Bull. 2011, 38, 789–796. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Lamentowicz, M.; Payne, R.J.; Mazei, Y. Effect of Taxonomic Resolution on Ecological and Palaeoecological Inference-A Test Using Testate Amoeba Water Table Depth Transfer Functions. Quat. Sci. Rev. 2014, 91, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Mooney, S.D.; Tinner, W. The Analysis of Charcoal in Peat and Organic Sediments. Mires Peat 2010, 7, 9. [Google Scholar]
- Finsinger, W.; Bonnici, I. Tapas: An R Package to Perform Trend and Peaks Analysis. Zenodo. Available online: https://hal.inria.fr/hal-03607000/ (accessed on 27 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: http://www.r-project.org/index.html(accessed on 27 December 2022).
- Simpson, G.L.; Oksanen, J. analogue: Analogue Matching and Modern Analogue Technique Transfer Function Models. (R Package Version 0.17-6). Available online: https://cran.r-project.org/package=analogue (accessed on 27 December 2022).
- Juggins, S. rioja: Analysis of Quaternary Science Data, R Package Version (1.0-5). Available online: https://cran.r-project.org/package=rioj (accessed on 27 December 2022).
- Grimm, E.C. CONISS: A FORTRAN 77 Program for Stratigraphically Constrained Cluster Analysis by the Method of Incremental Sum of Squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Bennett, K.D. Determination of the Number of Zones in a Biostratigraphical Sequence. New Phytol. 1996, 132, 155–170. [Google Scholar] [CrossRef]
- Panin, A.; Fuzeina, Y.; Karevskaya, I.; Sheremetskaya, E. Mid-Holocene Gullying Indicating Extreme Hydroclimatic Events in the Centre of the Russian Plain. Geogr. Pol. 2011, 84, 95–115. [Google Scholar] [CrossRef] [Green Version]
- Alexandrovskiy, A.; Ershova, E.; Ponomarenko, E.; Krenke, N.; Skripkin, V. Floodplain Paleosols of Moskva River Basin: Chronology and Paleoenvironment. Radiocarbon 2018, 60, 1169–1184. [Google Scholar] [CrossRef]
- Ershova, E.G.; Alexandrovskiy, A.L.; Krenke, N.A.; Korkishko, D.V. New Pollen Data from Paleosols in the Moskva River Floodplain (Nikolina Gora): Natural and Anthropogenic Environmental Changes during the Holocene. Quat. Int. 2016, 420, 294–305. [Google Scholar] [CrossRef]
- Lozovskaya, O. (Ed.) Site Zamostje 2 and Landscape Evolution in the Volga-Oka Region during the Holocene; IHMC RAS: St. Petersburg, Russia, 2018. [Google Scholar]
- Marcisz, K.; Tinner, W.; Colombaroli, D.; Kołaczek, P.; Słowiński, M.; Fiałkiewicz-Kozieł, B.; Łokas, E.; Lamentowicz, M. Long-Term Hydrological Dynamics and Fire History over the Last 2000 Years in CE Europe Reconstructed from a High-Resolution Peat Archive. Quat. Sci. Rev. 2015, 112, 138–152. [Google Scholar] [CrossRef]
- Sillasoo, Ü.; Väliranta, M.; Tuittila, E.-S. Fire History and Vegetation Recovery in Two Raised Bogs at the Baltic Sea: Fire History and Vegetation Recovery in Bogs. J. Veg. Sci. 2011, 22, 1084–1093. [Google Scholar] [CrossRef]
- Khotinsky, N. Holocene of the Northern Eurasia; Nauka: Moscow, Russia, 1977; 198p. (In Russian) [Google Scholar]
- Miagkaia, A.; Ershova, E. A 10,000-Year Pollen and Plant Macrofossil Record from the Losiny Ostrov National Park (Moscow, Russia). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 438, p. 012018. [Google Scholar]
- Borisova, O. Environmental and climatic conditions of human occupation in the central East European Plain during the Middle Holocene: Reconstruction from palaeofloristic data. Quat. Int. 2018, 516, 42–57. [Google Scholar] [CrossRef]
- Ershova, E.G.; Lozovskaya, O.M. Paleoenvrionemt of Mesolithic and Neolithic settlements at Zamostje 2 according to botanical and pollen analysis. In Site Zamostje 2 and Landscape Evolution in the Volga-Oka Region during the Holocene; IHMC RAS: St. Petersburg, Russia, 2018; pp. 31–40. [Google Scholar]
- Khotinsky, N.A. Athropogenic Changes in the Landscapes of the Russian Plain during the Holocene. Grana 1993, 32, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Kremenetskii, K.V.; Klimanov, V.A.; Boettgher, T.; Junge, F. The Climate of the Middle Volga Region in the Late Glaciation and Holocene. Dokl. Earth Sci. 2000, 370, 139–142. [Google Scholar]
- Bobrovsky, M.V.; Kupriaynov, D.A.; Khanina, L.G. Anthracological and Morphological Analysis of Soils for the Reconstruction of the Forest Ecosystem History (Meshchera Lowlands, Russia). Quat. Int. 2019, 516, 70–82. [Google Scholar] [CrossRef]
- Chernov, S.Z.; Ershova, E.G. Internal colonization in Russia during the 13th and 14th centuries: Three hamlets of the pre-manorial period. In Hierarchies in Rural Settlements; Klápštš, J., Ed.; Brepols: Turnhout, Belgium, 2013; pp. 387–406. [Google Scholar]
- Swan, J.; Gill, A. The Origins, Spread, and Consolidation of a Floating Bog in Harvard Pond, Petersham, Massachusetts. Ecology 1970, 51, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Abramova, L.I. Formation of Vegetation on Excavated Mires. Ph.D. Thesis, Lomonosov Moscow State University, Moscow, Russia, 1970. (In Russian). [Google Scholar]
- Kausch, A.P.; Seago, J.L., Jr.; Marsh, L.C. Changes in Starch Distribution in the Overwintering Organs of Typha latifolia (Typhaceae). Am. J. Bot. 1981, 68, 877–880. [Google Scholar] [CrossRef]
- Wilcox, D.A.; Simonin, H.A. The Stratigraphy and Development of a Floating Peatland, Pinhook Bog, Indiana. Wetlands 1988, 8, 75–91. [Google Scholar] [CrossRef]
- Smolders, A.J.P.; Tomassen, H.B.M.; van Mullekom, M.; Lamers, L.P.M.; Roelofs, J.G.M. Mechanisms Involved in the Re-Establishment of Sphagnum-dominated Vegetation in Rewetted Bog Remnants. Wetl. Ecol. Manag. 2003, 11, 403–418. [Google Scholar] [CrossRef]
- Gałka, M.; Lamentowicz, Ł.; Lamentowicz, M. Palaeoecology of Sphagnum obtusum in NE Poland. Bryologist 2013, 116, 238–247. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Tsyganov, A.N. Species Composition, Spatial Distribution and Seasonal Dynamics of Testate Amoebae Community in Sphagnum Bog (Middle Volga Region, Russia). Protistology 2007, 5, 156–206. [Google Scholar]
- Heger, T.J.; Mitchell, E.A.D.; Leander, B.S. Holarctic Phylogeography of the Testate Amoeba Hyalosphenia papilio (Amoebozoa: Arcellinida) Reveals Extensive Genetic Diversity Explained More by Environment than Dispersal Limitation. Mol. Ecol. 2013, 22, 5172–5184. [Google Scholar] [CrossRef]
- Weiner, A.K.M.; Cullison, B.; Date, S.V.; Tyml, T.; Volland, J.-M.; Woyke, T.; Katz, L.A.; Sleith, R.S. Examining the Relationship Between the Testate Amoeba Hyalosphenia papilio (Arcellinida, Amoebozoa) and Its Associated Intracellular Microalgae Using Molecular and Microscopic Methods. Protist 2022, 173, 125853. [Google Scholar] [CrossRef]
- Borzenok, L.I. Dynamics of Vegetation Cover of Mires in Moscow Region Meshchora during Anthopogenesis. Ph.D. Thesis, MOPU, Moscow, Russia, 2005. (In Russian). [Google Scholar]









| Laboratory Code | Depth, cm | Material | Age (14C yr BP) | Calibrated Dates, BCE/CE (95% Confidence Interval) |
|---|---|---|---|---|
| Undisturbed peat deposits | ||||
| UOC-16875 | 51 | peat | 1246 ± 36 | 675–752 CE (44.8%) |
| 757–775 CE (7.7%) | ||||
| 754–878 (42.3%) | ||||
| UOC-16874 | 101 | peat | 477 ± 26 | 1412–1451 CE (94.4%) |
| UOC-16873 | 163 | sapropel | 3548 ± 45 | 1979–1750 BCE (4.9%) |
| 2019–1996 BCE (90.1%) | ||||
| UOC-18280 | 175 | sapropel | 3984 ± 18 | 2498–2466 BCE (41.7%) |
| 2569–2521 BCE (53.1%) | ||||
| Floating vegetation mat | ||||
| GZ-10240 | 36 | peat | 145 ± 25 | 1670–1710 CE (15.6%) |
| 1719–1780 CE (23.9%) | ||||
| 1797–1824 CE (10.5%) | ||||
| 1832–1892 CE (26.4%) | ||||
| 1905–1945 CE (18.4%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazei, Y.A.; Tsyganov, A.N.; Ershova, E.G.; Mazei, N.G.; Pimenov, V.E.; Kotlyarova, E.V.; Kuzmenkova, N.V.; Paramonov, M.S.; Chulei, A.D.; Makarova, A.D.; et al. Multi-Proxy Paleoecological Reconstruction of Peatland Initiation, Development and Restoration in an Urban Area (Moscow, Russia). Diversity 2023, 15, 448. https://doi.org/10.3390/d15030448
Mazei YA, Tsyganov AN, Ershova EG, Mazei NG, Pimenov VE, Kotlyarova EV, Kuzmenkova NV, Paramonov MS, Chulei AD, Makarova AD, et al. Multi-Proxy Paleoecological Reconstruction of Peatland Initiation, Development and Restoration in an Urban Area (Moscow, Russia). Diversity. 2023; 15(3):448. https://doi.org/10.3390/d15030448
Chicago/Turabian StyleMazei, Yuri A., Andrey N. Tsyganov, Ekaterina G. Ershova, Natalia G. Mazei, Valery E. Pimenov, Elizaveta V. Kotlyarova, Natalia V. Kuzmenkova, Mikhail S. Paramonov, Artemii D. Chulei, Anastasiya D. Makarova, and et al. 2023. "Multi-Proxy Paleoecological Reconstruction of Peatland Initiation, Development and Restoration in an Urban Area (Moscow, Russia)" Diversity 15, no. 3: 448. https://doi.org/10.3390/d15030448