Impacts of Climate Change on Densities of the Urchin Centrostephanus rodgersii Vary among Marine Regions in Eastern Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection Methods
2.2. Centrostephanus rodgersii (Urchin) Density Modelling
- Water temperature at the sampling depth (Tz). Water temperature at depth was selected as a potential explanatory variable, rather than sea surface temperature (SST), as substantial variations in temperature occur with depth within the depth range where urchins are present [16]. Temperature is a key driver of C. rodgersii biological processes, including reproduction and larval survival [33,34]. Water temperatures (Tz) for model development were calculated as the mean of average monthly temperatures in February (i.e., summer maximum) over the period of 2002–2009. Summer temperatures were used as these have been shown to be a more powerful predictor of C. rodgersii densities than annual average temperatures [16]. Water temperatures at sampling depths were extracted from the E.U. Copernicus Marine Service (http://marine.copernicus.eu (accessed on 2 December 2022)), using the Global Ocean Physics Reanalysis monthly mean product (PHY_001_030). Data at the sampling depths were extracted at the closest depth available in the oceanographic re-analysis product;
- Water depth at the sampling site (Depth). Depth was selected as a potential explanatory variable as depth influences a range of factors including pressure, light, and wave exposure and is correlated to C. rodgersii densities [16]. Depth was recorded at each transect;
- Sampling date (Date). Date was included as a potential explanatory variable to allow the investigation of whether changes in C. rodgersii densities occurred over time. Date was incorporated as Julian day numbers throughout the study period;
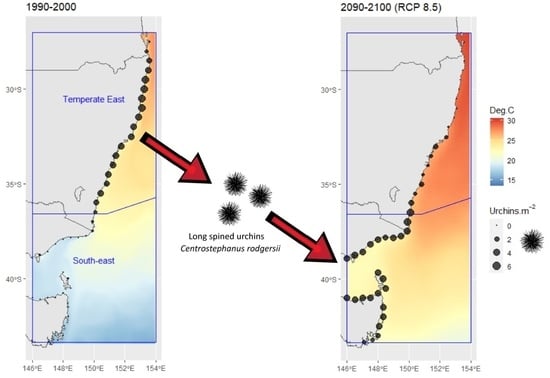
- Australian marine region (Region). The region was included as a potential explanatory variable to test the hypothesis that changes in C. rodgersii densities over time varied among distinct marine regions. To achieve this, the effects of Date on urchin densities were examined separately in each of the marine regions present. The study area encompassed sections of two distinct marine regions, the Temperate East region and South-east region (Figure 1a), as defined by Richardson et al. [20].
2.3. Historical C. rodgersii Urchin Density Predictions and Climate Change Projections
- Predictions for past C. rodgersii densities were made using average summer temperatures at depth (1–40 m), using data from the aforementioned oceanographic reanalysis product for the period 1990–2000;
- Predictions for nominal current C. rodgersii densities were made based on average summer temperatures at depth, using data from the same oceanographic reanalysis product for the period 2010–2020;
- Projections for future C. rodgersii densities, under RCP 8.5, were made using projected future average summer temperature at depth, for the period 2090–2100.
3. Results
3.1. Reef Life Survey and ATRC Urchin Data
3.2. Key Drivers of C. rodgersii Density Variations
3.3. Predicted and Projected C. rodgersii Densities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N. Climate Change Impacts on Marine Ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Babcock, R.C.; Bustamante, R.H.; Fulton, E.A.; Fulton, D.J.; Haywood, M.D.E.; Hobday, A.J.; Kenyon, R.; Matear, R.J.; Plaganyi, E.E.; Richardson, A.J. Severe Continental-Scale Impacts of Climate Change Are Happening Now: Extreme Climate Events Impact Marine Habitat Forming Communities along 45% of Australia’s Coast. Front. Mar. Sci. 2019, 6, 411. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Scheibling, R.E. Sea Urchin Barrens as Alternative Stable States of Collapsed Kelp Ecosystems. Mar. Ecol. Prog. Ser. 2014, 495, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp Forest Ecosystems: Biodiversity, Stability, Resilience and Future. Environ. Conserv. 2002, 29, 436–459. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.D. Range Expansion of a Habitat-Modifying Species Leads to Loss of Taxonomic Diversity: A New and Impoverished Reef State. Oecologia 2008, 156, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.J.; Liggins, L.; Banks, S.C.; Beheregaray, L.B.; Liddy, M.; McCulloch, G.A.; Waters, J.M.; Carter, L.; Byrne, M.; Cumming, R.A. The Population Genetic Structure of the Urchin Centrostephanus Rodgersii in New Zealand with Links to Australia. Mar. Biol. 2021, 168, 138. [Google Scholar] [CrossRef]
- Andrew, N.L. Spatial Heterogeneity, Sea Urchin Grazing, and Habitat Structure on Reefs in Temperate Australia. Ecology 1993, 74, 292–302. [Google Scholar] [CrossRef]
- Fletcher, W.J. Interactions among Subtidal Australian Sea Urchins, Gastropods, and Algae: Effects of Experimental Removals. Ecol. Monogr. 1987, 57, 89–109. [Google Scholar] [CrossRef]
- Byrne, M.; Andrew, N.L. Centrostephanus Rodgersii and Centrostephanus Tenuispinus. Dev. Aquac. Fish. Sci. 2020, 43, 379–396. [Google Scholar]
- Byrne, M.; Gall, M.L.; Campbell, H.; Lamare, M.D.; Holmes, S.P. Staying in Place and Moving in Space: Contrasting Larval Thermal Sensitivity Explains Distributional Changes of Sympatric Sea Urchin Species to Habitat Warming. Glob. Chang. Biol. 2022, 28, 3040–3053. [Google Scholar] [CrossRef]
- Johnson, C.R.; Ling, S.D.; Ross, D.J.; Shepherd, S.; Miller, K.J. Establishment of the Long-Spined Sea Urchin (Centrostephanus Rodgersii) in Tasmania: First Assessment of Potential Threats to Fisheries; School of Zoology and Tasmanian Aquaculture and Fisheries Institute: Hobart, Australia, 2005. [Google Scholar]
- Glasby, T.M.; Gibson, P.T. Decadal Dynamics of Subtidal Barrens Habitat. Mar. Environ. Res. 2020, 154, 104869. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J.; Kingsford, M.J.; Andrew, N.L. Patterns in Shallow Subtidal Marine Assemblages along the Coast of New South Wales. Aust. J. Ecol. 1991, 16, 231–249. [Google Scholar] [CrossRef]
- Andrew, N.L.; Underwood, A.J. Associations and Abundance of Sea Urchins and Abalone on Shallow Subtidal Reefs in Southern New South Wales. Mar. Freshw. Res. 1992, 43, 1547–1559. [Google Scholar] [CrossRef]
- Davis, T.R.; Cadiou, G.; Champion, C.; Coleman, M.A. Environmental Drivers and Indicators of Change in Habitat and Fish Assemblages within a Climate Change Hotspot. Reg. Stud. Mar. Sci. 2020, 36, 101295. [Google Scholar] [CrossRef]
- Davis, T.R.; Champion, C.; Coleman, M.A. Climate Refugia for Kelp within an Ocean Warming Hotspot Revealed by Stacked Species Distribution Modelling. Mar. Environ. Res. 2021, 166, 105267. [Google Scholar] [CrossRef]
- Andrew, N.L.; Underwood, A.J. Patterns of Abundance of the Sea Urchin Centrostephanus Rodgersii (Agassiz) on the Central Coast of New South Wales, Australia. J. Exp. Mar. Biol. Ecol. 1989, 131, 61–80. [Google Scholar] [CrossRef]
- Andrew, N.L. Changes in Subtidal Habitat Following Mass Mortality of Sea Urchins in Botany Bay, New South Wales. Aust. J. Ecol. 1991, 16, 353–362. [Google Scholar] [CrossRef]
- Keane, J.P.; Ling, S.D. Range Extension of the Long Spined Sea Urchin-Centrostephanus Rodgersii. In Proceedings of the SE Australia MCIA Symposium, CSIRO, Hobart, Australia, 20–21 February 2018. [Google Scholar]
- Richardson, A.J.; Eriksen, R.; Moltmann, T.; Hodgson-Johnston, I.; Wallis, J.R. State and Trends of Australia’s Ocean Report; Integrated Marine Observing System: Hobart, Australia, 2020. [Google Scholar]
- Duran, E.R.; England, M.H.; Spence, P. Surface Ocean Warming around Australia Driven by Interannual Variability and Long-term Trends in Southern Hemisphere Westerlies. Geophys. Res. Lett. 2020, 47, e2019GL086605. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8. 5 Tracks Cumulative CO2 Emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Edgar, G.J.; Cooper, A.; Baker, S.C.; Barker, W.; Barrett, N.S.; Becerro, M.A.; Bates, A.E.; Brock, D.; Ceccarelli, D.M.; Clausius, E. Reef Life Survey: Establishing the Ecological Basis for Conservation of Shallow Marine Life. Biol. Conserv. 2020, 252, 108855. [Google Scholar] [CrossRef]
- Edgar, G.J.; Barrett, N.S. Short Term Monitoring of Biotic Change in Tasmanian Marine Reserves. J. Exp. Mar. Biol. Ecol. 1997, 213, 261–279. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D. Ecological Effects of Marine Protected Areas on Rocky Reef Communities-a Continental-Scale Analysis. Mar. Ecol. Prog. Ser. 2009, 388, 51–62. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D. Systematic Global Assessment of Reef Fish Communities by the Reef Life Survey Program. Sci. Data 2014, 1, 140007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuur, A.F.; Saveliev, A.A.; Leno, E.N. Zero Inflated Models and Generalized Linear Mixed Models with R; Highland Statistics Ltd.: Newburgh, UK, 2012; ISBN 0957174101. [Google Scholar]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; de Clerck, O. Bio-ORACLE: A Global Environmental Dataset for Marine Species Distribution Modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Johnston, A.; Hochachka, W.; Strimas-Mackey, M.; Gutierrez, V.R.; Robinson, O.; Miller, E.; Auer, T.; Kelling, S.; Fink, D. Best Practices for Making Reliable Inferences from Citizen Science Data: Case Study Using EBird to Estimate Species Distributions. BioRxiv 2019, 574392, 1–13. [Google Scholar]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pecorino, D.; Lamare, M.D.; Barker, M.F.; Byrne, M. How Does Embryonic and Larval Thermal Tolerance Contribute to the Distribution of the Sea Urchin Centrostephanus Rodgersii (Diadematidae) in New Zealand? J. Exp. Mar. Biol. Ecol. 2013, 445, 120–128. [Google Scholar] [CrossRef]
- Byrne, M.; Andrew, N.L.; Worthington, D.G.; Brett, P.A. Reproduction in the Diadematoid Sea Urchin Centrostephanus Rodgersii in Contrasting Habitats along the Coast of New South Wales, Australia. Mar. Biol. 1998, 132, 305–318. [Google Scholar] [CrossRef]
- Hill, N.A.; Blount, C.; Poore, A.G.B.; Worthington, D.; Steinberg, P.D. Grazing Effects of the Sea Urchin Centrostephanus Rodgersii in Two Contrasting Rocky Reef Habitats: Effects of Urchin Density and Its Implications for the Fishery. Mar. Freshw. Res. 2003, 54, 691–700. [Google Scholar] [CrossRef]
- Davis, T.R.; Champion, C.; Coleman, M.A. Ecological Interactions Mediate Projected Loss of Kelp Biomass under Climate Change. Divers. Distrib. 2022, 28, 306–317. [Google Scholar] [CrossRef]
- Banks, S.C.; Piggott, M.P.; Williamson, J.E.; Bové, U.; Holbrook, N.J.; Beheregaray, L.B. Oceanic Variability and Coastal Topography Shape Genetic Structure in a Long-dispersing Sea Urchin. Ecology 2007, 88, 3055–3064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrego, D.; Howells, E.J.; Smith, S.D.A.; Madin, J.S.; Sommer, B.; Schmidt-Roach, S.; Cumbo, V.R.; Thomson, D.P.; Rosser, N.L.; Baird, A.H. Factors Limiting the Range Extension of Corals into High-Latitude Reef Regions. Diversity 2021, 13, 632. [Google Scholar] [CrossRef]
- Collin, R.; Rebolledo, A.P.; Smith, E.; Chan, K.Y.K. Thermal Tolerance of Early Development Predicts the Realized Thermal Niche in Marine Ectotherms. Funct. Ecol. 2021, 35, 1679–1692. [Google Scholar] [CrossRef]
- Andrew, N. Under Southern Seas: The Ecology of Australia’s Rocky Reefs; UNSW Press: Sydney, Australia, 1999; ISBN 0868406570. [Google Scholar]
- Kingsford, M.; Byrne, M. NSW Rocky Reefs Are under Threat. Mar. Freshw. Res. 2023, in press. [Google Scholar] [CrossRef]




| Model | Variables | AIC | ΔAIC | Deviance Explained |
|---|---|---|---|---|
| 1 | Date:Region + Depth + Tz | 20,276.8 | 0.0 | 44.3% |
| 2 | Depth + Tz | 20,436.9 | 160.1 | 42.2% |
| 3 | Tz | 20,704.1 | 427.3 | 38.9% |
| Variables | Effective Degrees of Freedom | p-Value |
|---|---|---|
| Tz | 4.948 | <0.001 |
| Depth | 4.828 | <0.001 |
| Date:Region (Temperate East) | 1.012 | 0.496 |
| Date:Region (South-east) | 4.234 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, T.R.; Knott, N.A.; Champion, C.; Przeslawski, R. Impacts of Climate Change on Densities of the Urchin Centrostephanus rodgersii Vary among Marine Regions in Eastern Australia. Diversity 2023, 15, 419. https://doi.org/10.3390/d15030419
Davis TR, Knott NA, Champion C, Przeslawski R. Impacts of Climate Change on Densities of the Urchin Centrostephanus rodgersii Vary among Marine Regions in Eastern Australia. Diversity. 2023; 15(3):419. https://doi.org/10.3390/d15030419
Chicago/Turabian StyleDavis, Tom R., Nathan A. Knott, Curtis Champion, and Rachel Przeslawski. 2023. "Impacts of Climate Change on Densities of the Urchin Centrostephanus rodgersii Vary among Marine Regions in Eastern Australia" Diversity 15, no. 3: 419. https://doi.org/10.3390/d15030419