Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources
- Accepted name;
- Plant family;
- Current chorology for indigenous plants or native range for exotic ones according to POWO [83];
- Period of introduction in Italy (i.e., archaeophyte or neophyte) for exotic plants;
- Data report in the study area;
- Only for exotic taxa, the current invasiveness status for each region, assessed by population monitoring over time according to the terminology of Pyšek et al. [84];
- Additional notes, including possible negative impacts of exotic taxa on habitats of interest to the European Community (Habitat Directive 92/43/EEC, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31992L0043, accessed on 10 November 2021).
2.3. Data Analysis
- Rtn = the richness of total native taxa (including cryptogenics) (n.);
- Rta = the richness of total alien taxa (including casual, naturalized, invasive and not assessed) (n.);
- FP = the degree of floristic pollution (i.e., total alien taxa/total flora × 100) (%);
- Ren = the richness of exclusive native taxa (i.e., regional endemics + taxa with wider distribution but reported in only one Italian region) (n.; %);
- Rea = the richness of exclusive alien taxa (n.; %);
- Ree = the richness of exclusive endemics (i.e., regional endemics) (n.; %);
- Dtn = the density of total native taxa (i.e., number of total native taxa/area) (taxa/km2);
- Dta = the density of total alien taxa (i.e., number of total native taxa/area) (taxa/km2);
- DNtn = the normalized density of total native taxa (i.e., natural logarithm of the number of total native taxa/natural logarithm of area) (taxa/km2);
- DNta = the normalized density of total alien taxa (i.e., natural logarithm of the number of total native taxa/natural logarithm of area) (taxa/km2).
3. Results
3.1. Floristic List
- Acanthus mollis L. subsp. mollis
- Acanthaceae—Hemicryptophyta scaposa—Mediterranean area (?)
- Change of status for Campania: from cryptogenic to invasive alien
- Actinidia deliciosa (A.Chev.) C.F.Liang & A.R.Ferguson
- Actinidiaceae—Phanerophyta lianosa—China—Neophyte
- First record for Campania (casual alien)
- Allium schoenoprasum L. subsp. schoenoprasum
- Amaryllidaceae—Geophyta bulbosa—Subcosmopolitan
- First record for Marche (casual alien)
- First record for Umbria (casual alien)
- Amaryllis belladonna L.
- Amaryllidaceae—Geophyta bulbosa—South Africa—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Andrachne telephioides L.
- Phyllanthaceae—Chamaephyta suffrutescentia—Mediterranean–South-East Asia
- New distribution data for Italian rare species in Lazio (native)
- New distribution data for Italian rare species in Campania (native)
- Antirrhinum majus L. subsp. majus
- Plantaginaceae—Chamaephyta fruticosa—South-West Europe (?)—Archaeophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Araujia sericifera Brot.
- Apocynaceae—Phanerophyta lianosa—South America—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Arctotheca calendula (L.) Levyns
- Asteraceae—Therophyta scaposa—South Africa—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Artemisia campestris L. subsp. campestris
- Asteraceae—Chamaephyta suffrutescentia—Subcosmopolitan
- Confirmation for Abruzzo (native)
- Asparagus setaceus (Kunth) Jessop
- Asparagaceae—Phanerophyta lianosa—East and South Africa—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Astragalus penduliflorus Lam.
- Fabaceae—Hemicryptophyta scaposa—European Orophyte
- New distribution data for Italian rare species in Lazio (native)
- Bidens formosa (Bonato) Sch.Bip.
- Asteraceae—Therophyta scaposa—North America—Neophyte
- First record for Lazio (casual alien)
- Bidens frondosa L.
- Asteraceae—Therophyta scaposa—North America—Neophyte
- Change of status for Toscana: from naturalized alien to invasive alien
- Bidens subalternans DC.
- Asteraceae—Therophyta scaposa—South America—Neophyte
- Change of status for Puglia: from naturalized alien to invasive alien
- Bidens tinctoria (Nutt.) Baill. ex Daydon
- Asteraceae—Therophyta scaposa—North and Central America—Neophyte
- First record for Marche (casual alien)
- Boerhavia coccinea Mill.
- Nyctaginaceae—Chamaephyta fruticosa—North, Central and South America—Neophyte
- First record for Campania (naturalized alien)
- Broussonetia papyrifera (L.) Vent.
- Moraceae—Phanerophyta caespitosa—South and South-East Asia—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Campanula portenschlagiana Schult.
- Campanulaceae—Hemicryptophyta scaposa—Balkans—Neophyte
- First record for Marche (casual alien)
- Change of status for Toscana: from casual alien to naturalized alien
- Canna indica L.
- Cannaceae—Geophyta rhizomatosa—North, Central and South America—Neophyte
- First record for Umbria (casual alien)
- Cenchrus setaceus (Forssk.) Morrone
- Poaceae—Hemicryptophyta caespitosa—North and East Africa and the Arabian Peninsula—Neophyte
- First record for Campania (naturalized alien)
- Centaurium erythraea Rafn subsp. rhodense (Boiss. & Reut.) Melderis
- Gentianaceae—Hemicryptophyta biennia—Steno-Mediterranean
- Confirmation for Basilicata (native)
- Ceratochloa cathartica (Vahl) Herter
- Poaceae—Hemicryptophyta caespitosa—South America—Neophyte
- First record for Basilicata (casual alien)
- Cerinthe retorta Sm.
- Boraginaceae—Therophyta scaposa—East Steno-Mediterranean
- New distribution data for Italian rare species in Puglia (native)
- Chamaecyparis lawsoniana (A.Murray) Parl.
- Cupressaceae—Phanerophyta scaposa—North America—Neophyte
- First record for Marche (casual alien)
- First record for Umbria (casual alien)
- Chasmanthe floribunda (Salisb.) N.E.Br.
- Iridaceae—Geophyta bulbosa—South Africa—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Chimonanthus praecox (L.) Link
- Calycanthaceae—Phanerophyta caespitosa—China—Neophyte
- First record for Marche (casual alien)
- Chlorophytum comosum (Thunb.) Jacques
- Asparagaceae—Hemicryptophyta scaposa—Africa—Neophyte
- First record for Umbria (casual alien)
- First record for Lazio (casual alien)
- Coleus scutellarioides (L.) Benth.
- Lamiaceae—Hemicryptophyta scaposa—South-East Asia and Australia—Neophyte
- First record for Calabria (casual alien)
- Cortaderia selloana (Schult. & Schult.f.) Asch. & Graebn.
- Poaceae—Hemicryptophyta caespitosa—South America—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Crassula muscosa L.
- Crassulaceae—Chamaephyta suffrutescentia—South Africa—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Crepis sancta (L.) Bornm. subsp. nemausensis (P.Fourn.) Babc.
- Asteraceae—Therophyta scaposa—East Mediterranean—Neophyte
- Change of status for Toscana: from naturalized alien to invasive alien
- Cuscuta campestris Yunck.
- Convolvulaceae—Therophyta parasitica—North, Central and South America—Neophyte
- Change of status for Umbria: from casual alien to naturalized alien
- Cyperus difformis L.
- Cyperaceae—Therophyta caespitosa—Africa, Europa (?) and South Asia—Neophyte
- Change of status for Toscana: from casual alien to naturalized alien
- Cyperus rotundus L.
- Cyperaceae—Geophyta rhizomatosa—Subcosmopolitan (?)
- Change of status for Calabria: from naturalized alien to cryptogenic
- Dactyloctenium aegyptium (L.) Willd.
- Poaceae—Therophyta scaposa—Africa and South Asia—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Datura inoxia Mill.
- Solanaceae—Therophyta scaposa—North, Central and South America—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Datura stramonium L.
- Solanaceae—Therophyta scaposa—North and Central America—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Delphinium hispanicum Willk. ex Costa
- Ranunculaceae—Therophyta scaposa—Eurasian—Neophyte
- First geolocalized reports in Puglia (naturalized alien)
- Deschampsia cespitosa (L.) P.Beauv. subsp. parviflora (Thuill.) Dumort.
- Poaceae—Hemicryptophyta caespitosa—Eurasian
- First record for Abruzzo (native)
- Digitaria ciliaris (Retz.) Koeler
- Poaceae—Therophyta scaposa—Africa and South Asia—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Drymochloa drymeja (Mert. & W.D.J.Koch) Holub subsp. exaltata (C.Presl) Foggi & Signorini
- Poaceae—Geophyta rhizomatosa—Italian endemic
- New distribution data for Italian rare species in Lazio (native)
- Echinochloa hispidula (Retz.) Nees
- Poaceae—Therophyta scaposa—Asia—Neophyte
- First record for Toscana (casual alien)
- Elaeagnus × submacrophylla Servett.
- Eleagnaceae—Nano-Phanerophyta—Japan and Korea—Neophyte
- First record for Toscana (casual alien)
- Elaeodendron croceum (Thunb.) DC.
- Celastraceae—Phanerophyta caespitosa—South Africa—Neophyte
- First record for Europe (casual alien)
- First record for Sardegna (casual alien)
- Eleusine indica (L.) Gaertn.
- Poaceae—Therophyta scaposa—Africa, South Asia and North Oceania—Neophyte
- Change of status for Toscana: from naturalized alien to invasive alien
- Eruca vesicaria (L.) Cav.
- Brassicaceae—Therophyta scaposa—Mediterranean (?) and Asian
- Change of status for Calabria: from naturalized alien to cryptogenic
- Erythrostemon gilliesii (Wall. ex Hook.) Klotzsch
- Fabaceae—Phanerophyta caespitosa—South America—Neophyte
- First record for Umbria (casual alien)
- Euphorbia cuneifolia Guss.
- Euphorbiaceae—Therophyta scaposa—West Mediterranean
- First geolocalized reports in Puglia (native)
- Euphorbia maculata L.
- Euphorbiaceae—Therophyta scaposa—North and Central America—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Euphorbia prostrata Aiton
- Euphorbiaceae—Therophyta scaposa—North, Central and South America—Neophyte
- Change of status for Umbria: from casual alien to naturalized alien
- Ficus microcarpa L.f.
- Moraceae—Phanerophyta scaposa—South-East Asia and Australia—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Galium lucidum All. subsp. lucidum
- Rubiaceae—Hemicryptophyta scaposa—Euri-Mediterranean
- First record for Puglia (native)
- Gladiolus italicus Mill.
- Iridaceae—Geophyta bulbosa—Mediterranean area (?) and South-West Asian
- Change of status for Calabria: from naturalized alien to cryptogenic
- Hydrangea macrophylla (Thunb.) Ser.
- Hydrangeaceae—Nano-Phanerophyta—Japan—Neophyte
- First record for Campania (casual alien)
- Hypericum × inodorum Mill.
- Hypericaceae—Nano-Phanerophyta—South and West European
- First record for Marche (native)
- Impatiens balfourii Hook.f.
- Balsaminaceae—Therophyta scaposa—South Asia—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Ipomoea indica (Burm.) Merr.
- Convolvulaceae—Geophyta rhizomatosa—North, Central and South America—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Ipomoea purpurea (L.) Roth
- Convolvulaceae—Therophyta scaposa—North, Central and South America—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Change of status for Basilicata: from invasive alien to naturalized alien
- Change of status for Calabria: from casual alien to naturalized alien
- Isatis tinctoria L. subsp. tinctoria
- Brassicaceae—Hemicryptophyta biennia—South-East Europe—Archaeophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Juglans nigra L.
- Juglandaceae—Phanerophyta scaposa—North America—Neophyte
- First record for Toscana (casual alien)
- Juglans regia L.
- Juglandaceae—Phanerophyta scaposa—South-West Asia—Neophyte (cryptogenic?)
- First record for Puglia (casual alien)
- Change of status for Calabria: from casual alien to naturalized alien
- Kalanchoë blossfeldiana Poelln.
- Crassulaceae—Hemicryptophyta succulenta—Madagascar—Neophyte
- First record for Europe (casual alien)
- First record for Sardegna (casual alien)
- Kalanchoë daigremontiana Raym.-Hamet & H.Perrier
- Crassulaceae—Chamaephyta succulenta—Madagascar—Neophyte
- Exclusion for Calabria (naturalized alien)
- Kalanchoë delagoënsis Eckl. & Zeyh.
- Crassulaceae—Chamaephyta succulenta—Madagascar—Neophyte
- First record for Campania (casual alien)
- Kalanchoë × houghtonii D.B.Ward
- Crassulaceae—Chamaephyta succulenta—Artificial origin—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Kalanchoë laxiflora Baker
- Crassulaceae—Hemicryptophyta succulenta—Madagascar—Neophyte
- Confirmation for Europe (casual alien)
- Confirmation for Italy (casual alien)
- First record for Basilicata (casual alien)
- Lamarckia aurea (L.) Moench
- Poaceae—Therophyta scaposa—Mediterranean-Turanian
- First geolocalized reports in Campania (native)
- Lantana camara L. subsp. aculeata (L.) R.W.Sanders
- Verbenaceae—Nano-Phanerophyta—North and Central America—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Lepidium virginicum L. subsp. virginicum
- Brassicaceae—Therophyta scaposa—North and Central America—Neophyte
- Change of status for Umbria: from casual alien to naturalized alien
- Leucaena leucocephala (Lam.) de Wit subsp. glabrata (Rose) Zárate
- Fabaceae—Phanerophyta scaposa—North and Central America—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Ligustrum lucidum W.T.Aiton
- Oleaceae—Phanerophyta scaposa—South-East Asia—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Melia azedarach L.
- Meliaceae—Phanerophyta scaposa—South-East Asia and Australia—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Morus nigra L.
- Moraceae—Phanerophyta scaposa—South-West Asia—Archaeophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Nephrolepis cordifolia (L.) C.Presl
- Nephrolepidaceae—Geophyta rhizomatosa—South-East Asia and Australia—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Nicotiana glauca Graham
- Solanaceae—Nano-Phanerophyta—South America—Neophyte
- Change of status for Puglia: from invasive alien to naturalized alien
- Nothoscordum gracile (Aiton) Stearn
- Amaryllidaceae—Geophyta bulbosa—Central and South America—Neophyte
- Change of status for Sardegna: from casual alien to naturalized alien
- Ophrys dinarica Kranjčev & P.Delforge
- Orchidaceae—Geophyta bulbosa—Amphi-Adriatic
- First geolocalized reports in Molise (native)
- Opuntia dillenii (Ker Gawl.) Haw.
- Cactaceae—Nano-Phanerophyta succulenta—North and Central America—Neophyte
- First record for Marche (naturalized alien)
- Opuntia ficus-indica (L.) Mill.
- Cactaceae—Phanerophyta succulenta—North America—Neophyte
- Change of status for Basilicata: from naturalized alien to invasive alien
- Oryza sativa L. subsp. sativa
- Poaceae—Therophyta scaposa—China—Archaeophyte
- Change of status for Toscana: from casual alien to naturalized alien
- Oxalis brasiliensis Lodd., G.Lodd. & W.Lodd. ex Drapiez
- Oxalidaceae—Geophyta bulbosa—South America—Neophyte
- First record for Europe (historical record for casual alien)
- First record for Italy (historical record for casual alien)
- First record for Toscana (historical record for casual alien)
- Oxalis debilis Kunth
- Oxalidaceae—Geophyta bulbosa—Central and South America—Neophyte
- First record for Friuli-Venezia Giulia (casual alien)
- First record for Umbria (casual alien)
- Oxalis dillenii Jacq.
- Oxalidaceae—Hemicryptophyta/Therophyta scaposa—North America—Neophyte
- Confirmation for Campania (casual alien)
- Oxalis latifolia Kunth
- Oxalidaceae—Geophyta bulbosa—North, Central and South America—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Oxalis pes-caprae L.
- Oxalidaceae—Geophyta bulbosa—South Africa—Neophyte
- First record for Veneto (casual alien)
- Papaver rhoeas L. subsp. rhoeas
- Papaveraceae—Therophyta scaposa—East-Mediterranean area (?)
- Change of status for Calabria: from naturalized alien to cryptogenic
- Parthenocissus inserta (A.Kern.) Fritsch
- Vitaceae—Phanerophyta lianosa—North America—Neophyte
- First record for Umbria (naturalized alien)
- First record for Calabria (casual alien)
- First record for Campania (naturalized alien)
- Parthenocissus tricuspidata (Siebold & Zucc.) Planch.
- Vitaceae—Phanerophyta lianosa—South-East Asia—Neophyte
- First record for Calabria (casual alien)
- Paspalum notatum Flüggé
- Poaceae—Hemicryptophyta caespitosa—South America—Neophyte
- First record for Campania (casual alien)
- Persicaria lapathifolia (L.) Delarbre subsp. lapathifolia
- Polygonaceae—Therophyta scaposa—Subcosmopolitan
- First record for Puglia (native)
- Phyllostachys aurea Carrière ex Rivière & C.Rivière
- Poaceae—Phanerophyta caespitosa—South-East Asia—Neophyte
- First record for Abruzzo (casual alien)
- First record for Molise (naturalized alien)
- Phyllostachys edulis (Carrière) J.Houz.
- Poaceae—Phanerophyta caespitosa—South-East Asia—Neophyte
- First record for Marche (naturalized alien)
- Physalis philadelphica Lam.
- Solanaceae—Therophyta scaposa—North and Central America—Neophyte
- First record for Campania (casual alien)
- Phytolacca americana L.
- Phytolaccaceae—Geophyta rhizomatosa—North America—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Platycladus orientalis (L.) Franco
- Cupressaceae—Phanerophyta caespitosa—East and South-East Asia—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Potentilla indica (Andrews) Th.Wolf
- Rosaceae—Hemicryptophyta rosulata—South and South-East Asia—Neophyte
- First record for Abruzzo (casual alien)
- Prunus avium (L.) L.
- Rosaceae—Phanerophyta scaposa—Europe, South-West Asia and North Africa (?)
- Change of status for Calabria: from naturalized alien to native
- Prunus laurocerasus L.
- Rosaceae—Phanerophyta scaposa/caespitosa—South-West Asia—Neophyte
- First record for Basilicata (casual alien)
- Rosa andegavensis Bastard
- Rosaceae—Nano-Phanerophyta—Europe and North-West Africa
- First record for Calabria (native)
- Rudbeckia hirta L.
- Asteraceae—Hemicryptophyta biennia—North America—Neophyte
- First record for Lazio (casual alien)
- Rumex patientia L. subsp. patientia
- Polygonaceae—Hemicryptophyta scaposa—Asia (?)—Archaeophyte
- Confirmation for Umbria (casual alien)
- Sedum palmeri S.Watson
- Crassulaceae—Chamaephyta succulenta—Central America—Neophyte
- Change of status for Toscana: from casual alien to naturalized alien
- Sedum spathulifolium Hook. var. spathulifolium
- Crassulaceae—Chamaephyta succulenta—North America—Neophyte
- First record for Europe (casual alien)
- First record for Marche (casual alien)
- First record for Umbria (casual alien)
- Senecio angulatus L.f.
- Asteraceae—Chamaephyta fruticosa—South Africa—Neophyte
- Change of the status for Calabria: from casual alien to naturalized alien
- Setaria italica (L.) P.Beauv. subsp. italica
- Poaceae—Therophyta scaposa—China—Archaeophyte
- Change of status for Calabria: from doubtful record to casual alien
- Setaria italica (L.) P.Beauv. subsp. pycnocoma (Steud.) de Wet
- Poaceae—Therophyta scaposa—Asia (?)—Neophyte
- Change of status for Marche: from casual alien to naturalized alien
- Solanum tuberosum L.
- Solanaceae—Geophyta rhizomatosa—South America—Neophyte
- Confirmation for Sicilia (casual alien)
- Solidago gigantea Aiton
- Asteraceae—Hemicryptophyta scaposa—North America—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Stenotaphrum secundatum (Walter) Kuntze
- Poaceae—Hemicryptophyta reptantia/Geophyta rhizomatosa—Tropical America and Africa—Neophyte
- First record for Basilicata (casual alien)
- Talinum paniculatum (Jacq.) Gaertn.
- Talinaceae—Chamaephyta suffrutescentia—North, Central and South America—Neophyte
- First record for Campania (casual alien)
- Trachelium caeruleum L. subsp. caeruleum
- Campanulaceae—Chamaephyta suffrutescentia—West Mediterranean—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Trachelospermum jasminoides (Lindl.) Lem.
- Apocynaceae—Phanerophyta lianosa—South-West Asia—Neophyte
- First record for Sardegna (casual alien)
- Trachycarpus fortunei (Hook.) H.Wendl.
- Arecaceae—Phanerophyta scaposa—South-East Asia—Neophyte
- Change of status for Toscana: from casual alien to naturalized alien
- Tradescantia fluminensis Vell.
- Commelinaceae—Hemicryptophyta reptantia/Geophyta rhizomatosa—South America—Neophyte
- Change of status for Calabria: from casual alien to naturalized alien
- Tradescantia sillamontana Matuda
- Commelinaceae—Hemicryptophyta reptantia/Geophyta rhizomatosa—North America—Neophyte
- First record for Sardegna (casual alien)
- Tropaeolum majus L.
- Tropaeolaceae—Therophyta reptantia—South America—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Ulmus pumila L.
- Ulmaceae—Phanerophyta scaposa—Asia—Neophyte
- Change of status for Lazio: from casual alien to naturalized alien
- Verbascum thapsus L. subsp. montanum (Schrad.) Bonnier & Layens
- Scrophulariaceae—Hemicryptophyta biennia—South-East European Orophyte
- Confirmation for Puglia (native)
- Veronica agrestis L.
- Plantaginaceae—Therophyta scaposa—European
- Confirmation for Puglia (native)
- Veronica cymbalaria Bodard subsp. cymbalaria
- Plantaginaceae—Therophyta scaposa—Euri-Mediterranean
- First record for Puglia (native)
- Veronica persica Poir.
- Plantaginaceae—Therophyta scaposa—South-West Asia—Neophyte
- Change of status for Calabria: from naturalized alien to invasive alien
- Vitis × koberi Ardenghi, Galasso, Banfi & Lastrucci
- Vitaceae—Phanerophyta lianosa—Artificial origin—Neophyte
- First record for Umbria (invasive alien)
- Vitis labrusca L.
- Vitaceae—Phanerophyta lianosa—North America—Neophyte
- First record for Marche (casual alien)
- Vitis rupestris Scheele
- Vitaceae—Phanerophyta lianosa—North America—Neophyte
- First record for Umbria (casual alien)
- Zephyranthes candida (Lindl.) Herb.
- Amaryllidaceae—Geophyta bulbosa—South America—Neophyte
- First record for Calabria (casual alien)
3.2. Current Floristic Knowledge in Italy
4. Discussion
4.1. New Findings and Updates
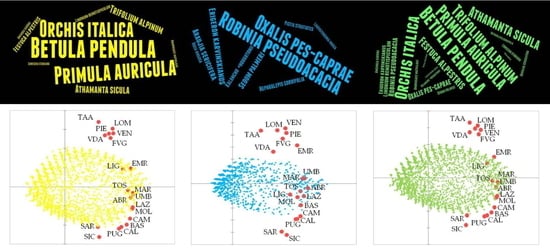
4.2. Exploration of Floristic Similarities between Regions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pignatti, S. Flora d’Italia 1–3; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Arcangeli, G. Compendio Della Flora Italiana; Ermanno Loercher: Torino, Italy, 1882. [Google Scholar]
- Arcangeli, G. Compendio Della Flora Italiana, 2nd ed.; Ermanno Loercher: Torino, Italy, 1894. [Google Scholar]
- Cesati, V.; Passerini, G.; Gibelli, G. Compendio della Flora Italiana 1–2; Dott. Francesco Villardi Tipografo-Editore: Milano, Italy, 1868–1886. [Google Scholar]
- Fiori, A.; Paoletti, G. Flora Analitica d’Italia 1–4; Tipografia del Seminario: Padova, Italy, 1896–1908. [Google Scholar]
- Fiori, A. Nuova Flora Analitica d’Italia 1–2; Tipografia M. Ricci: Firenze, Italy, 1923–1929. [Google Scholar]
- Zangheri, P. Flora Italica 1–2; Cedam: Padova, Italy, 1976. [Google Scholar]
- Bertoloni, A. Flora Italica 1–10; Typographaeo Richardi Masii: Bononiae, Italy, 1833–1854. [Google Scholar]
- Parlatore, F. Flora Italiana 1–5; Tipografia Le Monnier: Firenze, Italy, 1848–1875. [Google Scholar]
- Parlatore, F.; Caruel, T. Flora Italiana 6-10; Tipografia Le Monnier: Firenze, Italy, 1874–1896. [Google Scholar]
- Gottschlich, G. Hieracium boreoapenninum Gottschl. (Compositae), a new species from the Northern Apennine (Italy). Webbia 2009, 64, 3–7. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Salmeri, C. Hypericum scruglii sp. nov. (Guttiferae) from Sardinia. Nord. J. Bot. 2010, 28, 469–474. [Google Scholar] [CrossRef]
- Conti, F. A new species of Lathyrus L. (Fabaceae) from Central Apennine (Italy). Plant Biosyst. 2010, 144, 814–818. [Google Scholar] [CrossRef]
- Brullo, S.; Tomaselli, V.; Wagensommer, R.P. A new species of Odontites (Orobanchaceae) from southern Italy. Phytotaxa 2015, 213, 271–281. [Google Scholar] [CrossRef]
- Di Pietro, R. Taxonomic Features of Sesleria calabrica (Poaceae), a Neglected Species from Southern Italy. Folia Geobot. 2007, 42, 289–313. [Google Scholar] [CrossRef]
- Conti, F.; Giordano, C.; Moraldo, B.; Riccieri, C. Contributions to the Taxonomy of the Italian and Northern Balkanic Taxa in the Centaurea rupestris Group (Asteraceae). Ann. Bot. Fenn. 2011, 48, 193–218. [Google Scholar] [CrossRef]
- Dunkel, F.G. The Ranunculus auricomus L. complex (Ranunculaceae) in Central and Southern Italy, with additions to North Italian taxa. Webbia 2011, 66, 165–193. [Google Scholar] [CrossRef]
- Iamonico, D. Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Italy. Phytotaxa 2015, 199, 1–84. [Google Scholar] [CrossRef]
- Brullo, S.; Sciandrello, S. Cyperus alopecuroides Rottb. (Cyperaceae): Typification and first record for Sicily. Candollea 2006, 61, 365–372. [Google Scholar]
- Stinca, A.; Pignatti, S. A new combination in Smyrnium (Apiaceae). Phytotaxa 2016, 284, 137. [Google Scholar] [CrossRef]
- Banfi, E.; Galasso, G.; Bartolucci, F. Nomenclatural novelties for the Euro+Med flora. Nat. Hist. Sci. 2018, 5, 53–57. [Google Scholar] [CrossRef]
- Ito, Y.; Ohi-Toma, T.; Nepi, C.; Santangelo, A.; Stinca, A.; Tanaka, N.; Murata, J. Towards a better understanding of the Ruppia maritima complex (Ruppiaceae): Notes on the correct application and typification of the names R. cirrhosa and R. spiralis. Taxon 2017, 66, 167–171. [Google Scholar] [CrossRef]
- Wagensommer, R.P. Lectotypification of the name Genista michelii (Fabaceae). Phytotaxa 2017, 309, 99–100. [Google Scholar] [CrossRef]
- Conti, F.; Bartolucci, F.; Manzi, A.; Paolucci, M.; Santucci, B.; Petriccione, B.; Miglio, M.; Ciaschetti, G.; Stinca, A. Integrazioni alla flora vascolare dell’Italia Centrale. Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2016, 122, 33–42. [Google Scholar] [CrossRef]
- Salerno, G.; Stinca, A. First European record of Solandra maxima (Sessé & Moc.) P.S.Green (Solanaceae). Ann. Bot. 2017, 7, 67–70. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Bartolucci, F.; Fiorentino, M.; Licht, W.; Peccenini, S.; Perrino, E.V.; Venanzoni, R. First record for the flora of Italy and lectotypification of the name Linum elegans (Linaceae). Phytotaxa 2017, 296, 161–170. [Google Scholar] [CrossRef]
- Stinca, A.; Mei, G. Ehrharta erecta (Poaceae, Ehrhartoideae): Distribution in Italy and taxonomy of one of the most invasive plant species in the world. BioInvasions Rec. 2019, 8, 742–752. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 1; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 2; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 3; Edagricole: Bologna, Italy, 2018. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia 4; Edagricole: Bologna, Italy, 2019. [Google Scholar]
- Khandani, S.; Assadi, M.; Nejadsatari, T.; Mehregan, I. Phenetic analysis of the genera medicagoid Trigonella, Medicago and Melilotus (Fabaceae) on seed coat in Iran. Biodiversitas 2016, 17, 162–171. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Perrino, E.V.; Silletti, G.N.; Erben, M.; Wagensommer, R.P. Viola cassinensis subsp. lucana (Violaceae), a new subspecies from the Lucanian Apennine, southern Italy. Phyton 2018, 58, 109–115. [Google Scholar] [CrossRef]
- Rosati, L.; Coppi, A.; Farris, E.; Fascetti, S.; Becca, G.; Peregrym, M.; Tan, K.; Selvi, F. The genus Gymnospermium (Berberidaceae) in Italy: Identity and relationships of the populations at the western limit of the genus range. Plant Biosyst. 2019, 153, 796–808. [Google Scholar] [CrossRef]
- Sciandrello, S.; Galdo, G.; Salmeri, C.; Minissale, P. Vicia brulloi (Fabaceae), a new species from Sicily. Phytotaxa 2019, 418, 57–78. [Google Scholar] [CrossRef]
- Stinca, A.; Esposito, A. Typification of the name Stachys recta subsp. tenoreana (Lamiaceae). Phytotaxa 2019, 419, 110–112. [Google Scholar] [CrossRef]
- Stinca, A.; Conti, F.; Bartolucci, F. Typification of the name Centaurea deusta Ten. (Asteraceae). Phytotaxa 2019, 399, 296–299. [Google Scholar] [CrossRef]
- Musarella, C.M. Solanum torvum Sw. (Solanaceae): A new alien species for Europe. Genet. Resour. Crop Evol. 2020, 67, 515–522. [Google Scholar] [CrossRef]
- Rosati, L.; Fascetti, S.; Romano, V.A.; Potenza, G.; Lapenna, M.R.; Capano, A.; Nicoletti, P.; Farris, E.; de Lange, P.J.; Del Vico, E.; et al. New Chorological Data for the Italian Vascular Flora. Diversity 2020, 12, 22. [Google Scholar] [CrossRef]
- Stinca, A. Brugmansia suaveolens (Humb. & Bonpl. Ex Willd.) Sweet (Solanaceae): An alien species new to continental Europe. BioInvasions Rec. 2020, 9, 660–669. [Google Scholar] [CrossRef]
- Conti, F.; Bartolucci, F. Anthyllis apennina (Fabaceae), a new species from central Apennine (Italy). PhytoKeys 2021, 176, 111–129. [Google Scholar] [CrossRef]
- Di Pietro, R.; Kuzmanović, N.; Lakušić, D.; Viciani, D.; Fortini, P.; Iamonico, D. Nomenclatural and taxonomic notes on some names of Sesleria sect. Argenteae (Poaceae) from Italy and the Balkans. Phytotaxa 2021, 494, 89–102. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Venanzoni, R. Geranium lucarinii sp. nov. and re-evaluation of G. kikianum (Geraniaceae). Phytotaxa 2021, 489, 252–262. [Google Scholar] [CrossRef]
- Bartolucci, F.; Galasso, G.; Peruzzi, L.; Conti, F. Report 2020 on plant biodiversity in Italy: Native and alien vascular flora. Nat. Hist. Sci. 2021, 8, 41–54. [Google Scholar] [CrossRef]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Stinca, A.; Chianese, G.; D’Auria, G.; Fascetti, S.; Ravo, M.; Romano, V.A.; Salerno, G.; Astuti, G.; Bartolucci, F.; Bernardo, L.; et al. Contribution to the floristic knowledge of eastern Irpinia and Vulture-Melfese area (Campania and Basilicata, southern Italy). Ital. Bot. 2019, 8, 1–16. [Google Scholar] [CrossRef]
- Scoppola, A.; Blasi, C. (Eds.) Stato Delle Conoscenze Sulla Flora Vascolare d’italia; Palombi Editori: Roma, Italy, 2005. [Google Scholar]
- Williams, N.S.G.; Morgan, J.W.; Mcdonnell, M.J.; Mccarthy, M.A. Plant traits and local extinctions in natural grasslands along an urban–rural gradient. J. Ecol. 2005, 93, 1203–1213. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Zotti, M.; Bonanomi, G.; Cozzolino, A.; Stinca, A.; Migliozzi, A. Climatic and anthropogenic factors affect Ailanthus altissima invasion in a Mediterranean region. Plant Ecol. 2021, 222, 1347–1359. [Google Scholar] [CrossRef]
- Salinitro, M.; Alessandrini, A.; Zappi, A.; Tassoni, A. Impact of climate change and urban development on the flora of a southern European city: Analysis of biodiversity change over a 120-year period. Sci. Rep. 2019, 9, 9464. [Google Scholar] [CrossRef]
- Mihoub, J.B.; Henle, K.; Titeux, N.; Brotons, L.; Brummitt, N.A.; Schmeller, D.S. Setting temporal baselines for biodiversity: The limits of available monitoring data for capturing the full impact of anthropogenic pressures. Sci. Rep. 2017, 7, 41591. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Tarquini, S.; Isola, I.; Favalli, M.; Battistini, A. TINITALY, a Digital Elevation Model of Italy with a 10 Meters Cell Size (Version 1.0) [Data Set]; Istituto Nazionale di Geofisica e Vulcanologia (INGV). Available online: https://doi.org/10.13127/TINITALY/1.0 (accessed on 29 October 2021).
- ISTAT. Popolazione Residente al 1° Gennaio; National Institute of Statistics. Available online: http://dati.istat.it/Index.aspx?QueryId=18460 (accessed on 15 September 2021).
- Rivas-Martínez, S.; Penas, Á.; Díaz, T.E. Bioclimatic & Biogeographic Maps of Europe. Available online: http://www.globalbioclimatics.org/form/maps.htm (accessed on 7 September 2021).
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/ih/ (accessed on 15 September 2021).
- Bartolucci, F.; Domina, G.; Ardenghi, N.M.G.; Banfi, E.; Bernardo, L.; Bonari, G.; Buccomino, G.; Calvia, G.; Carruggio, F.; Cavallaro, V.; et al. Notulae to the Italian native vascular flora: 5. Ital. Bot. 2018, 5, 71–81. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Ardenghi, N.M.G.; Bacchetta, G.; Bernardo, L.; Buccomino, G.; Buono, S.; Caldararo, F.; Calvia, G.; Carruggio, F.; et al. Notulae to the Italian native vascular flora: 6. Ital. Bot. 2018, 6, 45–64. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Alessandrini, A.; Angiolini, C.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Bolpagni, R.; Bonari, G.; Bräuchler, C.; et al. Notulae to the Italian native vascular flora: 7. Ital. Bot. 2019, 7, 125–148. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Ardenghi, N.M.G.; Bacaro, G.; Bacchetta, G.; Ballarin, F.; Banfi, E.; Barberis, G.; Beccarisi, L.; Bernardo, L.; et al. Notulae to the Italian native vascular flora: 8. Ital. Bot. 2019, 8, 95–116. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Andreatta, S.; Angius, R.; Ardenghi, N.M.G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; Barberis, D.; Barberis, G.; et al. Notulae to the Italian native vascular flora: 9. Ital. Bot. 2020, 9, 71–86. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Bagella, S.; Barberis, G.; Briozzo, I.; Calbi, M.; Caria, M.C.; Cavallaro, V.; Chianese, G.; Cibei, C.; et al. Notulae to the Italian native vascular flora: 10. Ital. Bot. 2020, 10, 47–55. [Google Scholar] [CrossRef]
- Bartolucci, F.; Domina, G.; Andreatta, S.; Argenti, C.; Bacchetta, G.; Ballelli, S.; Banfi, E.; Barberis, D.; Barberis, G.; Bedini, G.; et al. Notulae to the Italian native vascular flora: 11. Ital. Bot. 2021, 11, 77–92. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Adorni, M.; Ardenghi, N.M.G.; Bonari, G.; Buono, S.; Cancellieri, L.; Chianese, G.; Ferretti, G.; Fiaschi, T.; et al. Notulae to the Italian alien vascular flora: 5. Ital. Bot. 2018, 5, 45–56. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Ballelli, S.; Bartolucci, F.; Brundu, G.; Buono, S.; Busnardo, G.; et al. Notulae to the Italian alien vascular flora: 6. Ital. Bot. 2018, 6, 65–90. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Ardenghi, N.M.G.; Aristarchi, C.; Bacchetta, G.; Bartolucci, F.; Bonari, G.; Bouvet, D.; Brundu, G.; Buono, S.; et al. Notulae to the Italian alien vascular flora: 7. Ital. Bot. 2019, 7, 157–182. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Andreatta, S.; Angiolini, C.; Ardenghi, N.M.G.; Aristarchi, C.; Arnoul, M.; Azzella, M.M.; Bacchetta, G.; Bartolucci, F.; et al. Notulae to the Italian alien vascular flora: 8. Ital. Bot. 2019, 8, 63–93. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Adorni, M.; Angiolini, C.; Apruzzese, M.; Ardenghi, N.M.G.; Assini, S.; Aversa, M.; Bacchetta, G.; Banfi, E.; et al. Notulae to the Italian alien vascular flora: 9. Ital. Bot. 2020, 9, 47–70. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Azzaro, D.; Bagella, S.; Barone, G.; Bartolucci, F.; Bianco, M.; Bolzani, P.; Bonari, G.; Boscutti, F.; et al. Notulae to the Italian alien vascular flora: 10. Ital. Bot. 2020, 10, 57–71. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Andreatta, S.; Argenti, E.; Bacchetta, G.; Bagella, S.; Banfi, E.; Barberis, D.; Bardi, S.; Barone, G.; et al. Notulae to the Italian alien vascular flora: 11. Ital. Bot. 2021, 11, 93–119. [Google Scholar] [CrossRef]
- Martellos, S.; Bartolucci, F.; Conti, F.; Galasso, G.; Moro, A.; Pennesi, R.; Peruzzi, L.; Pittao, E.; Nimis, P.L. FlorItaly—The portal to the Flora of Italy. PhytoKeys 2020, 156, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea 2; Cambridge University Press: Cambridge, UK, 1968. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea 3; Cambridge University Press: Cambridge, UK, 1972. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea 4; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentin, E.D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea 5; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Flora Europaea 1, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Flora of North America. Available online: http://www.efloras.org/flora_page.aspx?flora_id=1 (accessed on 10 September 2021).
- Flora of China. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 10 September 2021).
- Flora do Brasil 2020. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 10 September 2021).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 20 September 2021).
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Addinsoft 2021. XLSTAT Version 2021.3.1. Available online: https://www.xlstat.com/en/ (accessed on 15 September 2021).
- Ricciardi, M. Flora di Capri (Golfo di Napoli). Ann. Bot. 1998, 54, 7–169. [Google Scholar]
- Ricciardi, M.; Nazzaro, R.; Caputo, G.; De Natale, A.; Vallariello, G. The flora of the Island of Ischia (Bay of Naples). Webbia 2004, 59, 1–113. [Google Scholar] [CrossRef]
- Motti, R.; Ricciardi, M. The flora of the Phlegrean Fields (Gulf of Pozzuoli, Campania, Italy). Webbia 2005, 60, 395–476. [Google Scholar] [CrossRef]
- Stinca, A.; Motti, R. The vascular flora of the Royal Park of Portici (Naples, Italy). Webbia 2009, 64, 235–266. [Google Scholar] [CrossRef]
- Anzalone, B.; Iberite, B.; Lattanzi, E. La Flora vascolare del Lazio. Inform. Bot. Ital. 2010, 41, 187–317. [Google Scholar]
- Salerno, G. Segnalazioni Floristiche Italiane: 1010. Inform. Bot. Ital. 2001, 33, 37. [Google Scholar]
- De Natale, A. Note floristiche per il Parco Nazionale del Cilento e Vallo di Diano (Salerno, Campania). Inform. Bot. Ital. 2004, 36, 29–33. [Google Scholar]
- Stinca, A.; D’Auria, G.; Salerno, G.; Motti, R. Ulteriori integrazioni alla flora vascolare aliena della Campania (Sud Italia). Inform. Bot. Ital. 2013, 45, 71–81. [Google Scholar]
- Del Guacchio, E.; Magri, B. Notulae alla checklist della Flora vascolare Italiana 2: 1215. Inform. Bot. Ital. 2006, 38, 197. [Google Scholar]
- Nicolella, G. Noterella 0139. Acta Plant. Notes 2015, 3, 78. [Google Scholar]
- Lucchese, F. Atlante Della Flora Vascolare Del Lazio: Cartografia, Ecologia e Biogeografia. Vol. 1: Parte Generale e Flora Alloctona; Regione Lazio, Direzione Capitale Naturale, Parchi e Aree Protette: Roma, Italy, 2017. [Google Scholar]
- Parrella, G.; Greco, B.; Cennamo, G.; Griffo, R.; Stinca, A. Araujia sericifera new Host of Alfalfa mosaic virus in Italy. Plant Dis. 2013, 97, 1387. [Google Scholar] [CrossRef]
- Crisafulli, A.; Cannavò, S.; Maiorca, G.; Musarella, C.M.; Signorino, G.; Spampinato, G. Aggiornamenti floristici per la Calabria. Inform. Bot. Ital. 2010, 42, 437–448. [Google Scholar]
- Tison, J.-M.; de Foucault, B. Flora Gallica. Flore de France; Biotope: Mèze, France, 2014. [Google Scholar]
- Pirone, G.; Ciaschetti, G.; Di Martino, L.; Cianfaglione, K.; Giallonardo, T.; Frattaroli, A.R. Contribution to the knowledge of the coastal vegetation of Abruzzo (central Adriatic). Plant Sociol. 2014, 51 (Suppl. 1), 57–64. [Google Scholar] [CrossRef]
- Laface, V.L.A.; Musarella, C.M.; Cano Ortiz, A.; Quinto Canas, R.; Cannavò, S.; Spampinato, G. Three New Alien Taxa for Europe and a Chorological Update on the Alien Vascular Flora of Calabria (Southern Italy). Plants 2020, 9, 1181. [Google Scholar] [CrossRef]
- Lattanzi, E.; Tondi, G.; Di Pietro, R. Segnalazioni Floristiche Italiane: 909. Inform. Bot. Ital. 1998, 30, 64. [Google Scholar]
- Roma-Marzio, F.; D’Antraccoli, M.; Angeloni, D.; Bartolucci, F.; Bernardo, L.; Cancellieri, L.; Caruso, G.; Conti, F.; Dolci, D.; Gestri, G.; et al. Contribution to the floristic knowledge of Sillaro, Santerno, and Senio high valleys (Toscana, Italy). Ital. Bot. 2020, 10, 101–111. [Google Scholar] [CrossRef]
- Valdés, B. Boraginaceae . In Euro+Med Plantbase; Available online: http://ww2.bgbm.org/euroPlusMed/query.asp. (accessed on 10 September 2021).
- Wagensommer, R.P.; Fröhlich, T.; Fröhlich, M. First record of the southeast European species Cerinthe retorta Sibth. (Boraginaceae) in Italy and considerations on its distribution and conservation status. Acta Bot. Gall. 2014, 161, 111–115. [Google Scholar] [CrossRef]
- Karl, R.; Scholz, H. Med-Checklist Notulae, 28. Willdenowia 2009, 39, 335–345. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Perrino, E.V.; Silletti, G.N. Carex phyllostachys C.A. Mey. (Cyperaceae) new for Italy and phytogeographical considerations. Phyton 2014, 54, 215–222. [Google Scholar] [CrossRef]
- Terlevic, A.; Koopman, J.; Wieclaw, H.; Rešetnik, I.; Bogdanovic, S. Carex phyllostachys (Cyperaceae), a new species in Croatia. Acta Bot. Croat. 2021, 80, 106–111. [Google Scholar] [CrossRef]
- Fenu, G.; Bernardo, L.; Calvo, R.; Cortis, P.; De Agostini, A.; Gangale, C.; Gargano, D.; Gargano, M.L.; Lussu, M.; Medagli, P.; et al. Global and regional IUCN Red List assessments: 8. Ital. Bot. 2019, 8, 17–33. [Google Scholar] [CrossRef]
- Peruzzi, L.; Bedini, G. (Eds.) Wikiplantbase #Toscana v2.1. Available online: http://bot.biologia.unipi.it/wpb/toscana/index.html (accessed on 30 September 2021).
- Rojas-Sandoval, J.; Acevedo-Rodríguez, P. Cyperus rotundus . In Invasive Species Compendium; CAB International: Wallingford, UK; Available online: https://www.cabi.org/isc/datasheet/17506#todistribution (accessed on 1 November 2021).
- Amphlett, A. Deschampsia Cespitosa Subsp. Parviflora (Poaceae)—An under-recorded woodland grass. Br. Ir. Bot. 2019, 1, 117–127. [Google Scholar] [CrossRef]
- Wilhalm, T. Digitaria ciliaris in Europe. Willdenowia 2009, 39, 247–259. [Google Scholar] [CrossRef]
- Di Pietro, R.; Izco, J.; Blasi, C. Contribution to the nomenclatural knowledge of Fagus sylvatica woodlands of southern Italy. Plant Biosyst. 2004, 138, 27–36. [Google Scholar] [CrossRef]
- Di Pietro, R. Observations on the beech woodlands of the Apennines (peninsular Italy): An intricate biogeographical and syntaxonomical issue. Lazaroa 2009, 30, 89–97. [Google Scholar]
- Todini, A. Considerazioni sulla presenza a Roma di Festuca drymeia M. et K., specie di nuova segnalazione nel Lazio. Inform. Bot. Ital. 1999, 31, 39–42. [Google Scholar]
- Foggi, B.; Parolo, G.; Rossi, G.; Ardenghi, N.M.G.; Quercioli, C. Il genere Festuca e i generi affini per una flora critica dell’Italia. II. I generi Leucopoa e Drymochloa (Poaceae). Inform. Bot. Ital. 2010, 42, 335–361. [Google Scholar]
- Croce, A.; Stinca, A.; Santangelo, A.; Esposito, A. Exploring vascular flora biodiversity of two protected sandy coastal areas in southern Italy. Rend. Fis. Acc. Lincei 2019, 30, 323–336. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Ricciardi, M. Boschi misti costieri in Campania. Ann. Bot. 1995, 51 (Suppl. 10), 279–296. [Google Scholar]
- POWO. Elaeodendron croceum (Thunb.) DC. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:160659-1 (accessed on 20 September 2021).
- Mugnai, M.; Lazzaro, L.; Di Nuzzo, L.; Foggi, B.; Viciani, D.; Ferretti, G. Synopsis of Euphorbia section Anisophyllum (Euphorbiaceae) in Italy, with an insight on variation of distribution over time in Tuscany. Phytotaxa 2021, 485, 1–65. [Google Scholar] [CrossRef]
- Musarella, C.M.; Stinca, A.; Cano-Ortíz, A.; Laface, V.L.A.; Petrilli, R.; Esposito, A.; Spampinato, G. New data on the alien vascular flora of Calabria (southern Italy). Ann. Bot. 2020, 10, 55–66. [Google Scholar] [CrossRef]
- Licht, W.; Wagensommer, R.P. Flora Vascolare Del Gargano e Delle Isole Tremiti. Chiavi Analitiche per la Determinazione; Biblioteca Verde del Parco Nazionale del Gargano, Claudio Grenzi Ed.: Foggia, Italy, 2020. [Google Scholar]
- Fanfarillo, E.; Latini, L.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Salerno, G.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. 2020, 154, 935–946. [Google Scholar] [CrossRef]
- Caputo, G.; La Valva, V.; Nazzaro, R.; Ricciardi, M. La flora della Penisola Sorrentina (Campania). Delpinoa 1994, 31–32, 3–97. [Google Scholar]
- POWO. Hypericum ×inodorum Mill. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:433509-1 (accessed on 20 September 2021).
- Iamonico, D. Note floristiche per la Basilicata. Delpinoa 2006, 48, 21–27. [Google Scholar]
- Caruso, G.; Uzunov, D.; Gangale, C. Notulae alla checklist della Flora vascolare Italiana 4: 1215. 1410. Inform. Bot. Ital. 2007, 39, 433. [Google Scholar]
- Stinca, A.; Chianese, G.; D’Auria, G.; Del Guacchio, E.; Fascetti, S.; Perrino, E.V.; Rosati, L.; Salerno, G.; Santangelo, A. New alien vascular species for the flora of southern Italy. Webbia 2017, 72, 295–301. [Google Scholar] [CrossRef]
- Herrando-Moraira, S.; Vitales, D.; Nualart, N.; Gómez-Bellver, C.; Ibáñez, N.; Massó, S.; Cachón-Ferrero, P.; González-Gutiérrez, P.A.; Guillot, D.; Herrera, I.; et al. Global distribution patterns and niche modelling of the invasive Kalanchoe × houghtonii (Crassulaceae). Sci. Rep. 2020, 10, 3143. [Google Scholar] [CrossRef]
- Tenore, M. Flora Napolitana 3; Stamperia Francese: Napoli, Italy, 1824–1829. [Google Scholar]
- Tenore, M. Flora Medica Universale e Particolare Della Provincia di Napoli; Corso delle Botaniche Lezioni. Tipografia del giornale Enciclopedico: Napoli, Italy, 1923. [Google Scholar]
- Tenore, M. Sylloge Plantarum Vascularium Florae Napolitanae Hucusque Detectarum; Tipografia del Fibreno: Napoli, Italy, 1831. [Google Scholar]
- Galasso, G.; Domina, G.; Adorni, M.; Ardenghi, N.M.G.; Banfi, E.; Bedini, G.; Bertolli, A.; Brundu, G.; Calbi, M.; Cecchi, L.; et al. Notulae to the Italian alien vascular flora: 1. Ital. Bot. 2016, 1, 17–37. [Google Scholar] [CrossRef]
- Oliveri, N. Notulae alla flora esotica d’Italia 12: 271. Inform. Bot. Ital. 2015, 47, 77–90. [Google Scholar]
- Romolini, R.; Souche, R. Ophrys d’Italia; Éd. Sococor: Saint-Martin-de-Londres, France, 2012. [Google Scholar]
- Pezzetta, A. Le Orchidee del Molise. Giros Notizie 2015, 58, 71–87. [Google Scholar]
- Horne, H.E.; Barger, T.W.; Nesom, G.L. Two South American species of Oxalis (Oxalidaceae) naturalised in Alabama and the USA, first report. Phytoneuron 2013, 54, 1–7. [Google Scholar]
- Drapiez, F.M. Oxalis brasiliensis. Botanical Cabinet. In Encyclographie du Regne Vegetal; A L’Establissement Encyclographique: Bruxelles, Belgium, 1836; Volume 4, unpaged; Available online: https://books.google.it/books?id=ZjfOOTG0tPkC&printsec=frontcover&hl=it&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false (accessed on 20 September 2021).
- Hildebrand, F. Lebensverhältnisse der Oxalisarten; Verlag Von Gustav Fischer: Jena, Germany, 1884; pp. 43–46. Available online: https://books.google.it/books?id=WpM_AAAAYAAJ&pg=PA96&lpg=PA96&dq=Lebensverhaeltnisse+der+Oxalisarten+1884&source=bl&ots=4rF3UoX2Pj&sig=47MYQtRvESd9J0zF8uNehwu1TGs&hl=en&ei=G8CJTtLpFoH10gHjzszFDw&sa=X&oi=book_result&ct=result&sqi=2&redir_esc=y#v=onepage&q&f=false (accessed on 20 September 2021).
- IPNI. Oxalis brasiliensis G.Lodd. ex Drapiez. International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Botanic Gardens. Available online: https://www.ipni.org/n/77209116-1 (accessed on 20 September 2021).
- POWO. Oxalis brasiliensis G.Lodd. ex Drapiez. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:374712-1 (accessed on 20 September 2021).
- Jenny, R. The Botanical Cabinet. Lankesteriana 2008, 8, 43–52. [Google Scholar] [CrossRef]
- Stinca, A. Oxalidaceae. In Flora d’Italia; Pignatti, S., Guarino, R., La Rosa, M., Eds.; Edagricole: Bologna, Italy, 2017; Volume 2, pp. 353–360. [Google Scholar]
- Kadereit, J.W. Some suggestions on the geographical origin of the central, west and north European synanthropic species of Papaver L. Bot. J. Linn. Soc. 1990, 103, 221–231. [Google Scholar] [CrossRef]
- Lu, L.; Wen, J.; Chen, Z. A combined morphological and molecular phylogenetic analysis of Parthenocissus (Vitaceae) and taxonomic implications. Bot. J. Linn. Soc. 2012, 168, 43–63. [Google Scholar] [CrossRef]
- Moore, M.O.; Wen, J. Parthenocissus Planchon. In Flora of North America; 2016; Volume 12, pp. 16–18. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=124105 (accessed on 20 October 2021).
- Motti, R.; Stinca, A. Analysis of the biodeteriogenic vascular flora at the Royal Palace of Portici in southern Italy. Int. Biodeterior. Biodegrad. 2011, 65, 1256–1265. [Google Scholar] [CrossRef]
- Stinca, A.; Croce, A.; D’Auria, G.; Salerno, G.; Santangelo, A.; Rosati, L.; Motti, R. Nuovi dati sulla flora vascolare aliena della Campania (Sud Italia). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2016, 122, 89–110. [Google Scholar] [CrossRef]
- Stinca, A.; Galasso, G.; Banfi, E. First Italian record of Paspalum notatum Flüggé (Poaceae) and its typification. Acta Bot. Croat. 2016, 75, 153–156. [Google Scholar] [CrossRef]
- Spampinato, G.; Sciandrello, S.; Giusso Del Galdo, G.; Puglisi, M.; Tomaselli, V.; Cannavò, S.; Musarella, C.M. Contribution to the knowledge of Mediterranean wetland biodiversity: Plant communities of the Aquila Lake (Calabria, Southern Italy). Plant Sociol. 2019, 56, 53–68. [Google Scholar] [CrossRef]
- Parrella, G.; Troiano, E.; Stinca, A.; Pozzi, M.I. Molecular and serological detection of Parietaria mottle virus in Phytolacca americana, a new host of the virus. Phytopathol. Mediterr. 2021, 60, 101–104. [Google Scholar] [CrossRef]
- Ohba, H. Sedum Linnaeus. In Flora of North America; Oxford University Press: Oxford, UK, 2009; Volume 8, pp. 199–222. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=129989 (accessed on 20 October 2021).
- Giardina, G.; Raimondo, F.M.; Spadaro, V. A catalogue of plants growing in Sicily. Bocconea 2007, 20, 5–582. [Google Scholar]
- Stinca, A.; D’Auria, G.; Motti, R. Sullo status invasivo di Bidens bipinnata, Phoenix canariensis, Pistia stratiotes e Tradescantia fluminensis in Campania (Sud Italia). Inform. Bot. Ital. 2012, 44, 295–299. [Google Scholar]
- Stinca, A.; D’Auria, G.; Motti, R. Integrazioni alla flora vascolare aliena della Campania (Sud Italia). Inform. Bot. Ital. 2012, 44, 287–293. [Google Scholar]
- Curti, L.; Lorenzoni, G.G.; Marchiori, S. Florula del Bacino del Lago di Lesina (Foggia). Mem. Biogeogr. Adriat. 1974, 9, 45–117. [Google Scholar]
- Forte, L.; Cavallaro, V.; Pantaleo, F.; D’Amico, F.S.; Macchia, F. The vascular Flora of the “Bosco Isola” at Lesina (Foggia-Apulia). Fl. Medit. 2002, 12, 33–92. [Google Scholar]
- Ardenghi, N.M.G.; Galasso, G.; Banfi, E.; Zoccola, A.; Foggi, B.; Lastrucci, L. A taxonomic survey of the genus Vitis L. (Vitaceae) in Italy, with special reference to Elba Island (Tuscan Archipelago). Phytotaxa 2014, 166, 163–198. [Google Scholar] [CrossRef]
- Stinca, A. The genus Vitis L. (Vitaceae) in Campania (Southern Italy), with emphasis on alien units. Ann. di Bot. 2019, 9, 107–112. [Google Scholar] [CrossRef]
- Del Guacchio, E.; De Natale, A.; Stinca, A. Notes to the non-native flora of Campania (Southern Italy). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2020, 127, 39–49. [Google Scholar] [CrossRef]
- Mei, G.; Šegota, V.; Stinca, A.; Vukelić, J.; Baričević, D.; Taffetani, F.; Alegro, A. Cystopteris dickieana R.Sim (Cystopteridaceae), a new fern in the continental Balkans flora. Plant Biosyst. 2021, 155, 1–4. [Google Scholar] [CrossRef]
- González-Rodríguez, A.M.; Baruch, Z.; Palomo, D.; Cruz-Trujillo, G.; Jiménez, M.S.; Morales, D. Ecophysiology of the invader Pennisteum setaceum and three native grasses in the Canary Islands. Acta Oecol. 2010, 36, 248–254. [Google Scholar] [CrossRef]
- Pasta, S.; Badalamenti, E.; La Mantia, T. Tempi e modi di un’invasione incontrastata: Pennisetum setaceum (Forssk.) Chiov. (Poaceae) in Sicilia. Nat. Sicil. 2010, 34, 487–525. [Google Scholar]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, A.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K.; Touza, J.; Perrings, C.; Williamson, M. A century of the ornamental plant trade and its impact on invasion success. Divers. Distrib. 2007, 13, 527–534. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Kokkoris, Y.; Zikos, A.; Zervou, S.; Delipetrou, P.; Cardoso, A.C.; Deriu, I.; Gervasini, E.; et al. Alien plants of Europe: Introduction pathways, gateways and time trends. PeerJ 2021, 9, e11270. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M.; et al. Red list of threatened vascular plant species in Italy. Plant Biosyst. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- Rossi, G.; Orsenigo, S.; Gargano, D.; Montagnani, C.; Peruzzi, L.; Fenu, G.; Abeli, T.; Alessandrini, A.; Astuti, G.; Bacchetta, G.; et al. Lista Rossa Della Flora Italiana. 2 Endemiti e Altre Specie Minacciate; Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2020. [Google Scholar]
- Fenu, G.; Abdelaal, M.; Bacchetta, G.; Bongiorni, L.; Cogoni, A.; Cortis, P.; Croce, A.; Fois, M.; Lussu, M.; Perrino, E.V.; et al. Global and Regional IUCN Red List Assessments: 6. Ital. Bot. 2018, 6, 31–44. [Google Scholar] [CrossRef]
- Lang, P.L.M.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using herbaria to study global environmental change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Sassone, A.B. The Uses of Herbaria in Botanical Research. A Review Based on Evidence From Argentina. Front. Plant Sci. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Tison, J.-M.; de Foucault, B. Flora Gallica. Flore de France; Biotope Éditions: Mèze, France, 2014. [Google Scholar]
- Vangjeli, J. Excursion Flora of Albania; Koeltz Scientific Books: Königstein, Germany, 2015. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Aedo, C.; Buira, A.; Medina, L.; Fernández-Albert, M. The Iberian Vascular Flora: Richness, Endemicity and Distribution Patterns. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 101–130. [Google Scholar]
- Danihelka, J.; Chrtek, J., Jr.; Kaplan, Z. Checklist of vascular plants of the Czech Republic. Preslia 2012, 84, 647–811. [Google Scholar]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Arianoutsou, M.; Essl, F.; Hejda, M.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invasions 2010, 12, 3525–3549. [Google Scholar] [CrossRef]
- Domingues de Almeida, J.; Freitas, H. Exotic flora of continental Portugal—A new assessment. Bocconea 2012, 24, 231–237. [Google Scholar]
- Barina, Z.; Rakaj, M.; Somogyi, G.; Erős-Honti, Z.; Pifkó, D. The alien flora of Albania: History, current status and future trends. Weed Res. 2014, 54, 196–215. [Google Scholar] [CrossRef]
- Uludağ, A.; Aksoy, N.; Yazlık, A.; Arslan, Z.F.; Yazmış, E.; Üremiş, I.; Cossu, T.A.; Groom, Q.; Pergl, J.; Pyšek, P.; et al. Alien flora of Turkey: Checklist, taxonomic composition and ecological attributes. NeoBiota 2017, 35, 61–85. [Google Scholar] [CrossRef]
- Blasi, C.; Filibek, G.; Burrascano, S.; Copiz, R.; Di Pietro, R.; Ercole, S.; Lattanzi, E.; Rosati, L.; Tilia, A. Primi risultati per una nuova regionalizzazione fitogeografica del territorio italiano. Biogeographia 2007, 28, 9–23. [Google Scholar] [CrossRef]
- Pignatti, S. Ecologia del paesaggio; UTET: Torino, Italy, 1997. [Google Scholar]
- Peruzzi, L.; Conti, F.; Bartolucci, F. An inventory of vascular plants endemic to Italy. Phytotaxa 2014, 168, 1–75. [Google Scholar] [CrossRef]
- Abbate, G.; Scassellati, E.; Bonacquisti, S.; Iberite, M.; Latini, M.; Giuliani, A. Using woody genera for phytogeographic regionalization at a medium scale: A case study of Italy. Botany 2016, 94, 533–542. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Diniz-Filho, J.A.F. ‘Latitude’ and geographic patterns in species richness. Ecography 2004, 27, 268–272. [Google Scholar] [CrossRef]
- Kessler, M.; Grytnes, J.A.; Halloy, S.R.P.; Kluge, J.; Krömer, T.; León, B.; Macía, M.J.; Young, K.R. Gradients of Plant Diversity: Local Patterns and Processes. In Climate Change Effects on The Biodiversity of The Tropical Andes; Herzog, S.K., Martinez, R., Jorgensen, P.M., Tiessen, H., Eds.; Inter-American-Institute of Global Change Research: São José dos Campos, Brazil, 2011; pp. 204–219. [Google Scholar]
- Ohdo, T.; Takahashi, K. Plant species richness and community assembly along gradients of elevation and soil nitrogen availability. AoB Plants 2020, 12, plaa014. [Google Scholar] [CrossRef]
- Ramos, C.S.; Picca, P.; Pocco, M.E.; Filloy, J. Disentangling the role of environment in cross-taxon congruence of species richness along elevational gradients. Sci. Rep. 2021, 11, 4711. [Google Scholar] [CrossRef]
- Lazarina, M.; Charalampopoulos, A.; Psaralexi, M.; Krigas, N.; Michailidou, D.E.; Kallimanis, A.S.; Sgardelis, S.P. Diversity patterns of different life forms of plants along an elevational gradient in Crete, Greece. Diversity 2019, 11, 200. [Google Scholar] [CrossRef]
- Subedi, C.K.; Rokaya, M.B.; Münzbergová, Z.; Timsina, B.; Gurung, J.; Chettri, N.; Baniya, C.B.; Ghimire, S.K.; Chaudhary, R.P. Vascular plant diversity along an elevational gradient in the Central Himalayas, western Nepal. Folia Geobot. 2020, 55, 127–140. [Google Scholar] [CrossRef]
- Di Biase, L.; Pace, L.; Mantoni, C.; Fattorini, S. Variations in Plant Richness, Biogeographical Composition, and Life Forms along an Elevational Gradient in a Mediterranean Mountain. Plants 2021, 10, 2090. [Google Scholar] [CrossRef]
- Conti, F.; Uzunov, D. Crepis magellensis F. Conti & Uzunov (Asteraceae), a new species from Central Apennine (Abruzzo, Italy). Candollea 2011, 66, 81–86. [Google Scholar] [CrossRef]
- Brullo, S.; Pavone, P.; Salmeri, C. Allium aetnense (Amaryllidaceae), a new species from Sicily. Plant. Biosyst. 2013, 147, 835–843. [Google Scholar] [CrossRef]
- Španiel, S.; Kaplan, K.; Bovio, M.; Mártonfiová, L.; Cetlová, V. Alyssum rossetii (Brassicaceae), a new species from the Aosta Valley in Italy based on morphological and genome size data. Phytotaxa 2018, 360, 269–281. [Google Scholar] [CrossRef]
- Olden, J.D. Biotic homogenization: A new research agenda for conservation biogeography. J. Biogeogr. 2006, 33, 2027–2039. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; van Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef]





| Italian Regions | First Records (n.) | Confirmations (n.) | Exclusions (n.) | Changes of Status (n.) | First Geolocalized Reports (n) | Total (n.) |
|---|---|---|---|---|---|---|
| CAL | 5 | 1 | 1 | 37 | 44 | |
| UMB | 10 | 1 | 3 | 14 | ||
| TOS | 4 | 8 | 12 | |||
| MAR | 10 | 1 | 11 | |||
| CAM | 9 | 1 | 1 | 1 | 12 | |
| PUG | 4 | 2 | 2 | 2 | 10 | |
| LAZ | 3 | 7 | 10 | |||
| BAS | 4 | 1 | 2 | 7 | ||
| SAR | 4 | 1 | 5 | |||
| ABR | 3 | 1 | 4 | |||
| MOL | 1 | 1 | 2 | |||
| FVG | 1 | 1 | ||||
| VEN | 1 | 1 | ||||
| SIC | 1 | 1 | ||||
| Total (n.) | 59 | 8 | 1 | 62 | 4 | 134 |
| Italian Regions | Natives (n.) | Cryptogenics (n.) | TOTAL NATIVES (Rtn; n.) | Casual Aliens (n.) | Naturalized Aliens (n.) | Invasive Aliens (n.) | Aliens with No Indication of Invasiveness Status (n.) | TOTAL ALIENS (Rta; n.) | TOTAL FLORA (TOT. NATIVES + TOT. ALIENS) (n.) | Degree of Floristic Pollution (FP; %) | Exclusive Natives (Excluded the Species Reported as “Sensu Lato”) (Ren; n.) | Exclusive Aliens (Excluded the Species Reported as “Sensu Lato”) (Ran; n.) | Exclusive Endemics (Ree; n.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABR | 2900 | 20 | 2920 | 223 | 138 | 34 | 1 | 396 | 3316 | 11.94 | 91 | 0 | 71 |
| BAS | 2495 | 18 | 2513 | 142 | 80 | 21 | 4 | 247 | 2760 | 8.95 | 21 | 2 | 14 |
| CAL | 2496 | 18 | 2514 | 178 | 124 | 48 | 6 | 356 | 2870 | 12.40 | 86 | 7 | 65 |
| CAM | 2414 | 22 | 2436 | 237 | 149 | 49 | 0 | 435 | 2871 | 15.15 | 33 | 20 | 27 |
| EMR | 2582 | 22 | 2604 | 296 | 252 | 29 | 12 | 589 | 3193 | 18.45 | 16 | 17 | 4 |
| FVG | 2754 | 10 | 2764 | 288 | 239 | 37 | 77 | 641 | 3405 | 18.83 | 162 | 20 | 24 |
| LAZ | 2839 | 21 | 2860 | 318 | 166 | 41 | 1 | 526 | 3386 | 15.53 | 21 | 19 | 11 |
| LIG | 2631 | 16 | 2647 | 307 | 180 | 19 | 2 | 508 | 3155 | 16.10 | 64 | 41 | 10 |
| LOM | 2911 | 15 | 2926 | 548 | 288 | 118 | 1 | 955 | 3881 | 24.61 | 60 | 81 | 24 |
| MAR | 2326 | 19 | 2345 | 224 | 129 | 40 | 14 | 407 | 2752 | 14.79 | 23 | 6 | 19 |
| MOL | 2194 | 15 | 2209 | 95 | 85 | 26 | 5 | 211 | 2420 | 8.72 | 1 | 0 | 0 |
| PIE | 3004 | 24 | 3028 | 234 | 279 | 70 | 1 | 584 | 3612 | 16.17 | 213 | 26 | 46 |
| PUG | 2210 | 34 | 2244 | 240 | 126 | 21 | 0 | 387 | 2631 | 14.71 | 63 | 12 | 38 |
| SAR | 2223 | 32 | 2255 | 338 | 215 | 72 | 5 | 630 | 2885 | 21.84 | 357 | 43 | 259 |
| SIC | 2581 | 31 | 2612 | 221 | 221 | 18 | 10 | 470 | 3082 | 15.25 | 446 | 44 | 293 |
| TAA | 2757 | 21 | 2778 | 665 | 237 | 43 | 14 | 959 | 3737 | 25.66 | 201 | 72 | 75 |
| TOS | 3172 | 30 | 3202 | 311 | 242 | 65 | 6 | 624 | 3826 | 16.31 | 107 | 19 | 22 |
| UMB | 2073 | 17 | 2090 | 200 | 98 | 13 | 21 | 332 | 2422 | 13.71 | 4 | 1 | 2 |
| VDA | 1767 | 37 | 1804 | 85 | 73 | 21 | 1 | 180 | 1984 | 9.07 | 23 | 2 | 4 |
| VEN | 2808 | 14 | 2822 | 425 | 258 | 70 | 32 | 785 | 3607 | 21.76 | 43 | 18 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions. Diversity 2021, 13, 600. https://doi.org/10.3390/d13110600
Stinca A, Musarella CM, Rosati L, Laface VLA, Licht W, Fanfarillo E, Wagensommer RP, Galasso G, Fascetti S, Esposito A, et al. Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions. Diversity. 2021; 13(11):600. https://doi.org/10.3390/d13110600
Chicago/Turabian StyleStinca, Adriano, Carmelo Maria Musarella, Leonardo Rosati, Valentina Lucia Astrid Laface, Wolfgang Licht, Emanuele Fanfarillo, Robert Philipp Wagensommer, Gabriele Galasso, Simonetta Fascetti, Assunta Esposito, and et al. 2021. "Italian Vascular Flora: New Findings, Updates and Exploration of Floristic Similarities between Regions" Diversity 13, no. 11: 600. https://doi.org/10.3390/d13110600