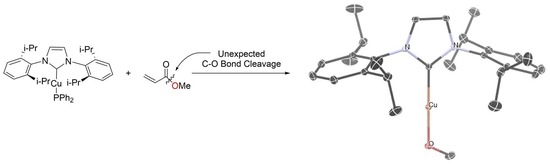
Unexpected C–O Bond Cleavage by a Copper–Phosphido Compound
Abstract
:1. Introduction
2. Results and Discussion

3. Experimental
3.1. General Considerations
3.2. Experimental Details
3.3. X-ray Structure Determinations
Crystal Data for C28H39CuN2O
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Danopoulos, A.A.; Simler, T.; Braunstein, P. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef] [PubMed]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper–NHC complexes in catalysis. Coord. Chem. Rev. 2015, 293–294, 48–79. [Google Scholar] [CrossRef] [Green Version]
- Horsley Downie, T.M.; Hall, J.W.; Collier Finn, T.P.; Liptrot, D.J.; Lowe, J.P.; Mahon, M.F.; McMullin, C.L.; Whittlesey, M.K. The first ring-expanded NHC–copper(i) phosphides as catalysts in the highly selective hydrophosphination of isocyanates. Chem. Commun. 2020, 56, 13359–13362. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Hou, Z. Highly efficient catalytic hydrosilylation of carbon dioxide by an N-heterocyclic carbene copper catalyst. Chem. Commun. 2013, 49, 4782–4784. [Google Scholar] [CrossRef]
- Bonet, A.; Lillo, V.; Ramírez, J.; Díaz-Requejo, M.M.; Fernández, E. The selective catalytic formation of β-boryl aldehydes through a base-free approach. Org. Biomol. Chem. 2009, 7, 1533–1535. [Google Scholar] [CrossRef]
- Nayal, O.S.; Hong, J.; Yang, Y.; Mo, F. Cu-Catalysed carboxylation of aryl boronic acids with CO2. Org. Chem. Front. 2019, 6, 3673–3677. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.W.; Unson, D.M.L.; Brunel, P.; Collins, L.R.; Cybulski, M.K.; Mahon, M.F.; Whittlesey, M.K. Copper-NHC-Mediated Semihydrogenation and Hydroboration of Alkynes: Enhanced Catalytic Activity Using Ring-Expanded Carbenes. Organometallics 2018, 37, 3102–3110. [Google Scholar] [CrossRef]
- Fortman, G.C.; Slawin, A.M.Z.; Nolan, S.P. A Versatile Cuprous Synthon: [Cu(IPr)(OH)] (IPr = 1, 3 bis(diisopropylphenyl)imidazol-2-ylidene). Organometallics 2010, 29, 3966–3972. [Google Scholar] [CrossRef]
- Coyle, J.P.; Sirianni, E.R.; Korobkov, I.; Yap, G.P.A.; Dey, G.; Barry, S.T. Study of Monomeric Copper Complexes Supported by N-Heterocyclic and Acyclic Diamino Carbenes. Organometallics 2017, 36, 2800–2810. [Google Scholar] [CrossRef]
- Dannenberg, S.G.; Waterman, R. A bench-stable copper photocatalyst for the rapid hydrophosphination of activated and unactivated alkenes. Chem. Commun. 2020, 56, 14219–14222. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, S.G.; Seth, D.M., Jr.; Finfer, E.J.; Waterman, R. Divergent Mechanistic Pathways for Copper(I) Hydrophosphination Catalysis: Understanding That Allows for Diastereoselective Hydrophosphination of a Tri-substituted Styrene. ACS Catal. 2023, 13, 550–562. [Google Scholar] [CrossRef]
- Ohishi, T.; Zhang, L.; Nishiura, M.; Hou, Z. Carboxylation of Alkylboranes by N-Heterocyclic Carbene Copper Catalysts: Synthesis of Carboxylic Acids from Terminal Alkenes and Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 8114–8117. [Google Scholar] [CrossRef] [PubMed]
- Goj, L.A.; Blue, E.D.; Munro-Leighton, C.; Gunnoe, T.B.; Petersen, J.L. Cleavage of X−H Bonds (X = N, O, or C) by Copper(I) Alkyl Complexes To Form Monomeric Two-Coordinate Copper(I) Systems. Inorg. Chem. 2005, 44, 8647–8649. [Google Scholar] [CrossRef]
- Waterman, R. Triamidoamine-Supported Zirconium Compounds in Main Group Bond-Formation Catalysis. Acc. Chem. Res. 2019, 52, 2361–2369. [Google Scholar] [CrossRef]
- Reuter, M.B.; Seth, D.M.; Javier-Jiménez, D.R.; Finfer, E.J.; Beretta, E.A.; Waterman, R. Recent advances in catalytic pnictogen bond forming reactions via dehydrocoupling and hydrofunctionalization. Chem. Commun. 2023, 59, 1258–1273. [Google Scholar] [CrossRef]
- Glueck, D.S. Metal-catalyzed nucleophilic carbon–heteroatom (C–X) bond formation: The role of M–X intermediates. Dalton Trans. 2008, 39, 5276–5286. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Toste, F.D. Non-Oxidative Vanadium-Catalyzed C–O Bond Cleavage: Application to Degradation of Lignin Model Compounds. Angew. Chem. Int. Ed. 2010, 49, 3791–3794. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Ammal, S.C.; Lin, Z.; You, K.-E.; Heyden, A.; Chen, J.G. Controlling reaction pathways of selective C–O bond cleavage of glycerol. Nat. Commun. 2018, 9, 4612. [Google Scholar] [CrossRef] [Green Version]
- Oyeyemi, V.B.; Keith, J.A.; Carter, E.A. Trends in Bond Dissociation Energies of Alcohols and Aldehydes Computed with Multireference Averaged Coupled-Pair Functional Theory. J. Phys. Chem. 2014, 118, 3039–3050. [Google Scholar] [CrossRef]
- Kratzert, D. FinalCif, V118. Available online: https://dkratzert.de/finalcif.html (accessed on 22 April 2023).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dannenberg, S.G.; Waterman, R. Unexpected C–O Bond Cleavage by a Copper–Phosphido Compound. Molbank 2023, 2023, M1659. https://doi.org/10.3390/M1659
Dannenberg SG, Waterman R. Unexpected C–O Bond Cleavage by a Copper–Phosphido Compound. Molbank. 2023; 2023(2):M1659. https://doi.org/10.3390/M1659
Chicago/Turabian StyleDannenberg, Steven G., and Rory Waterman. 2023. "Unexpected C–O Bond Cleavage by a Copper–Phosphido Compound" Molbank 2023, no. 2: M1659. https://doi.org/10.3390/M1659