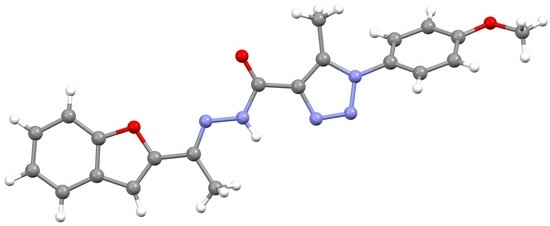
(E)-N’-(1-(Benzofuran-2-yl)ethylidene)-1-(4-Methoxyphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carbohydrazide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. IR and NMR Spectroscopy
2.3. Crystal Structure of 3
3. Materials and Methods
3.1. General
3.2. Synthesis of 3
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popiołek, Ł. Updated information on antimicrobial activity of hydrazide–hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; İmamoğlu, N. Design, synthesis, and anticancer evaluation of novel tetracaine hydrazide-hydrazones. ACS Omega 2023, 8, 9198–9211. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Uppal, G.; Kajal, A.; Kamboj, S.; Sharma, V. Hydrazones as promising lead with diversity in bioactivity-therapeutic potential in present scenario. Int. J. Pharm. Sci. Rev. Res. 2013, 18, 65–74. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A. Hydrazide-hydrazones of 3-methoxybenzoic acid and 4-tert-butylbenzoic acid with promising antibacterial activity against Bacillus spp. J. Enzym. Inhib. Med. Chem. 2016, 31, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popiołek, Ł.; Stefańska, J.; Kiełczykowska, M.; Musik, I.; Biernasiuk, A.; Malm, A.; Wujec, M. Synthesis, dissociation constants, and antimicrobial activity of novel 2,3-disubstituted-1,3-thiazolidin-4-one derivatives. J. Heterocycl. Chem. 2016, 53, 393–402. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Tang, X.; Song, L. Recent access to polycycles via post-Ugi reactions. Processes 2023, 11, 699. [Google Scholar] [CrossRef]
- Tang, X.; Ding, S.; Song, L.; Van der Eycken, E.V. Transition metal-catalyzed C-H activation/annulation approaches to isoindolo[2,1-b]isoquinolin-5(7H)-ones. Chem. Rec. 2023, 23, e202200255. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-Y.; Chen, Z.-A.; Shen, Q.-K.; Quan, Z.-S. Application of triazoles in the structural modification of natural products. J. Enzym. Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.A.; Abdel-Wahab, B.F.; El-Hiti, G.A. 2-Acetylbenzofurans: Synthesis, reactions and applications. Curr. Org. Chem. 2010, 14, 48–64. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodarahmi, G.; Asadi, P.; Hassanzadeh, F.; Khodarahmi, E. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: A review. J. Res. Med. Sci. 2015, 20, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef] [Green Version]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Mohamed, H.A.; Bekheit, M.S.; El-Hiti, G.A. Synthesis and characterization of novel 2-(1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles and 2-(4,5-dihydro-1H-pyrazol-1-yl)-4-(1H-1,2,3-triazol-4-yl)thiazoles. Molecules 2022, 27, 8904. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, B.M.; Abdel-Wahab, B.F.; Bekheit, M.S.; El-Hiti, G.A. Intermolecular interactions of 3,5-bis(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in a cocrystal with 1,3-bis(4-methoxyphenyl)prop-2-en-1-one and dimethylformamide Solvate. Crystals 2022, 12, 663. [Google Scholar] [CrossRef]
- Dong, H.-S.; Wang, B. Synthesis of some novel 3,6-bis(1,2,3-triazolyl)-s-triazolo[3,4-b]-1,3,4-thiadiazole derivatives. J. Chin. Chem. Soc. 2005, 52, 103–108. [Google Scholar] [CrossRef]
- Elliott, E.D. The preparation and properties of 2-vinylbenzofuran. J. Am. Chem. Soc. 1952, 73, 754. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Wahab, B.F.; Bekheit, M.S.; Kariuki, B.M.; El-Hiti, G.A. (E)-N’-(1-(Benzofuran-2-yl)ethylidene)-1-(4-Methoxyphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carbohydrazide. Molbank 2023, 2023, M1657. https://doi.org/10.3390/M1657
Abdel-Wahab BF, Bekheit MS, Kariuki BM, El-Hiti GA. (E)-N’-(1-(Benzofuran-2-yl)ethylidene)-1-(4-Methoxyphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carbohydrazide. Molbank. 2023; 2023(2):M1657. https://doi.org/10.3390/M1657
Chicago/Turabian StyleAbdel-Wahab, Bakr F., Mohamed S. Bekheit, Benson M. Kariuki, and Gamal A. El-Hiti. 2023. "(E)-N’-(1-(Benzofuran-2-yl)ethylidene)-1-(4-Methoxyphenyl)-5-Methyl-1H-1,2,3-Triazole-4-Carbohydrazide" Molbank 2023, no. 2: M1657. https://doi.org/10.3390/M1657