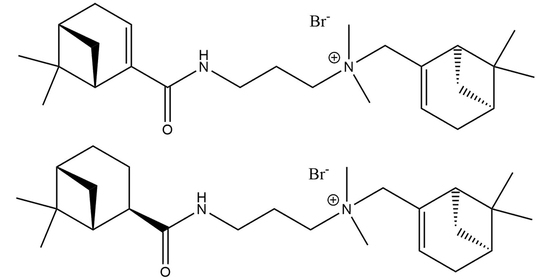
N-(((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)-3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamido)-N,N-dimethylpropan-1-aminium Bromide
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. General
3.2. General Procedure for Synthesis of (1R,5S)-N-(3-(dimethylamino)propyl)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamide 5, 6
3.3. General Procedure for Synthesis of N-(((1S,5R)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)-3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamido)-N,N-dimethylpropan-1-aminium bromide 9, 10
3.4. Antibacterial and Antifungal Activities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Si, H.; Wang, P.; Chen, S.; Shang, S.; Song, Z.; Wang, Z.; Liao, S. Derivatization of Natural Compound β-Pinene Enhances Its In Vitro Antifungal Activity against Plant Pathogens. Molecules 2019, 24, 3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.-S.; Duan, W.-G.; Yang, L.-X.; Huang, M.; Lei, F.-H. Synthesis and Antifungal Activity of Novel Myrtenal-Based 4-Methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.Y.; Trizna, E.Y.; Sulaiman, R.K.; Pavelyev, R.S.; Gilfanov, I.R.; Lisovskaya, S.A.; Ostolopovskaya, O.V.; Frolova, L.L.; Kutchin, A.V.; Guseva, G.B.; et al. Increasing the Efficacy of Treatment of Staphylococcus aureus–Candida albicans Mixed Infections with Myrtenol. Antibiotics 2022, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, M.N.; Kazakova, R.R.; Lubina, A.P.; Zeldi, M.I.; Nikitina, E.V.; Balakin, K.V.; Shtyrlin, Y.G. Antibacterial activity profile of miramistin in in vitro and in vivo models. Microb. Pathog. 2022, 142, 104072. [Google Scholar] [CrossRef] [PubMed]
- de Richter, R.K.; Bonato, M.; Follet, M.; Kamenka, J.M. The (+)-and (−)-[2-(1, 3-dithianyl)] myrtanylborane. Solid and stable monoalkylboranes for asymmetric hydroboration. J. Org. Chem. 1990, 55, 2855–2860. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tan, H.-B.; He, M.-T.; Li, L.; Wang, Y.-H.; Long, C.-L. Isolation and synthesis of two hydroxychavicol heterodimers from Piper nudibaccatum. Tetrahedron 2015, 71, 2369–2375. [Google Scholar] [CrossRef]
- Leclercq, R.; Canton, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Strains | MIC, µg/mL | ||||
|---|---|---|---|---|---|
| 9 | 10 | Miramistin | Ciprofloxacin | Fluconazole | |
| S. aureus ATCC29213 | 128 | 128 | 4 | 0.25 | ND |
| E. coli MG1655 | 512 | 512 | 8 | 2 | ND |
| Candida albicans NCTC- 885-653 | 750 | 1500 | >750 | ND | 46 |
| Candida tropicalis Y-1513/784 | 750 | 1500 | >1500 | ND | 94 |
| Aspergillus niger F-1119 | 750 | >1500 | >1500 | ND | >1500 |
| Rhizopus nigricans F-1537/1722 | >1500 | >1500 | >1500 | ND | >1500 |
| Fusarium oxysporum (clinical isolate) | >1500 | >1500 | >1500 | ND | >1500 |
| Trichophyton rubrum (clinical isolate) | >1500 | >1500 | >1500 | ND | >1500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilfanov, I.R.; Pavelyev, R.S.; Nikitina, L.E.; Frolova, L.L.; Popov, A.V.; Rakhmatullin, I.Z.; Klochkov, V.V.; Lisovskaya, S.A.; Trizna, E.Y.; Grishaev, D.Y.; et al. N-(((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)-3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamido)-N,N-dimethylpropan-1-aminium Bromide. Molbank 2023, 2023, M1592. https://doi.org/10.3390/M1592
Gilfanov IR, Pavelyev RS, Nikitina LE, Frolova LL, Popov AV, Rakhmatullin IZ, Klochkov VV, Lisovskaya SA, Trizna EY, Grishaev DY, et al. N-(((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)-3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamido)-N,N-dimethylpropan-1-aminium Bromide. Molbank. 2023; 2023(1):M1592. https://doi.org/10.3390/M1592
Chicago/Turabian StyleGilfanov, Ilmir R., Roman S. Pavelyev, Liliya E. Nikitina, Larisa L. Frolova, Alexey V. Popov, Ilfat Z. Rakhmatullin, Vladimir V. Klochkov, Svetlana A. Lisovskaya, Elena Yu. Trizna, Denis Yu. Grishaev, and et al. 2023. "N-(((1S,5R)-6,6-Dimethylbicyclo[3.1.1]hept-2-en-2-yl)methyl)-3-((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-ene/ane-2-carboxamido)-N,N-dimethylpropan-1-aminium Bromide" Molbank 2023, no. 1: M1592. https://doi.org/10.3390/M1592