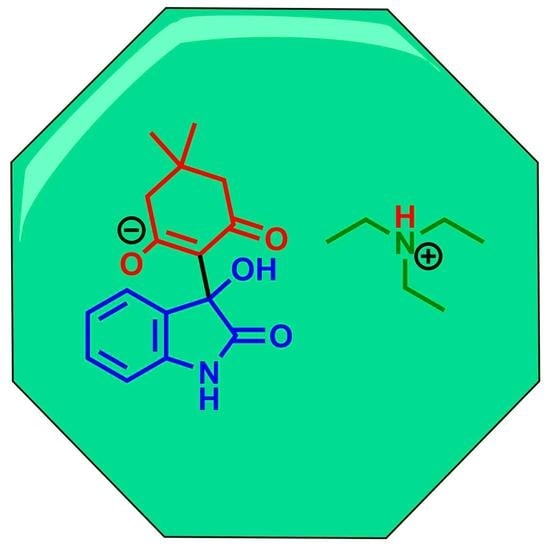
Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate 4
2.2. NMR Study of Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate 4 Structure
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zdrazil, B.; Guha, R. The Rise and Fall of a Scaffold: A Trend Analysis of Scaffolds in the Medicinal Chemistry Literature. J. Med. Chem. 2018, 61, 4688–4703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dietrich, J. Privileged scaffolds in lead generation. Expert Opin. Drug Discov. 2015, 10, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Skoreński, M.; Sieńczyk, M. The Fellowship of Privileged Scaffolds—One Structure to Inhibit Them All. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; Abouzied, A.S.; Abbas, N.S. Synthesis and Biological Evaluation of Novel 4,5,6,7-Tetrahydrobenzo[D]-Thiazol-2-Yl Derivatives Derived from Dimedone with Anti-Tumor, C-Met, Tyrosine Kinase and Pim-1 Inhibitions. Anticancer Agents Med. Chem. 2019, 19, 1438–1453. [Google Scholar] [CrossRef]
- Rao, T.N.; Krishnarao, N.; Ahmed, F.; Alomar, S.Y.; Albalawi, F.; Mani, P.; Aljaafari, A.; Parvatamma, B.; Arshi, N.; Kumar, S. One-Pot Synthesis of 7,7-Dimethyl-4-Phenyl-2-Thioxo-2,3,4,6,7,8-Hexahydro-1H-Quinazoline-5-OnesUsing Zinc Ferrite Nanocatalyst and Its Bio Evaluation. Catalysts 2021, 11, 431. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.M.; Al-Qahtany, B.M.; Ali, M.; Teleb, M.; Al-Agamy, M.H.; Naz, S.; Ul-Haq, Z. Synthesis, antimicrobial activity, pharmacophore modeling and molecular docking studies of new pyrazole-dimedone hybrid architectures. Chem. Central J. 2018, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Maharvi, G.M.; Ali, S.; Riaz, N.; Afza, N.; Malik, A.; Ashraf, M.; Iqbal, L.; Lateef, M. Mild and efficient synthesis of new tetraketones as lipoxygenase inhibitors and antioxidants. J. Enzyme Inhib. Med. Chem. 2008, 23, 62–69. [Google Scholar] [CrossRef]
- Melis, C.; Meleddu, R.; Angeli, A.; Distinto, S.; Bianco, G.; Capasso, C.; Cottiglia, F.; Angius, R.; Supuran, C.T.; Maccioni, E. Isatin: A privileged scaffold for the design of carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Jin, K.; Li, J.; Jiang, Y.; Li, X.; Wang, X.; Huang, Y.; Zhang, Y.; Xu, W. LJNK, an indoline-2,3-dione-based aminopeptidase N inhibitor with promising antitumor potency. Anti Cancer Drugs 2016, 27, 496–507. [Google Scholar] [CrossRef]
- Corona, A.; Meleddu, R.; Esposito, F.; Distinto, S.; Bianco, G.; Masaoka, T.; Maccioni, E.; Menéndez-Arias, L.; Alcaro, S.; Le Grice, S.F.; et al. Ribonuclease H/DNA Polymerase HIV-1 Reverse Transcriptase Dual Inhibitor: Mechanistic Studies on the Allosteric Mode of Action of Isatin-Based Compound RMNC6. PLoS ONE 2016, 11, e0147225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavari, M.; Malana, S.F.; Joubert, J. Design, synthesis, biological evaluation and docking studies of sulfonyl isatin derivatives as monoamine oxidase and caspase-3 inhibitors. Med. Chem. Commun. 2016, 7, 1628–1639. [Google Scholar] [CrossRef]
- Akdemir, A.; Güzel-Akdemir, Ö.; Karalı, N.; Supuran, C.T. Isatin analogs as novel inhibitors of Candida spp. β-carbonic anhydrase enzymes. Bioorg. Med. Chem. 2016, 24, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, Z.; He, D.; Yan, C.; Liu, W. Coumarin-Isatin Type Compound Useful in Treatment of Diabetes Mellitus and Its Preparation. Patent CN105237521A, 2016. Available online: https://patents.google.com/patent/CN105237521A/zh (accessed on 19 January 2023).
- Cristofoli, M.; Kung, C.-P.; Hadgraft, J.; Lane, M.E.; Sil, B.C. Ion Pairs for Transdermal and Dermal Drug Delivery: A Review. Pharmaceutics 2021, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; Available online: https://goldbook.iupac.org (accessed on 19 January 2023).
- Elinson, M.N.; Merkulova, V.M.; Ilovaisky, A.I.; Chizhov, A.O.; Belyakov, P.A.; Barba, F.; Batanero, B. Electrochemically induced aldol reaction of cyclic 1,3-diketones with isatins. Electrochim. Acta 2010, 55, 2129–2133. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Kalashnikova, V.M.; Egorov, M.P. Noncatalytic on water aldol reaction of isatins with cyclic 1,3-diketones at room temperature without the need for subsequent chromatography. Mendeleev Commun. 2022, 32, 543–545. [Google Scholar] [CrossRef]
- Pfitzinger, W. Chinolinderivate aus Isatinsäure. J. Prakt. Chem. 1886, 33, 100. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Fang, L.; Wang, P.; Lu, C.; Yan, F. A simple one-pot synthesis of quinoline-4-carboxylic acid derivatives by Pfitzinger reaction of isatin with ketones in water. Monatsh. Chem. 2013, 144, 391–394. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Fakhrutdinov, A.N.; Elinson, M.N. Ammonium Salts of 5-(3-Chromenyl)-5H-chromeno[2,3-b]pyridines. Molbank 2021, 2021, M1219. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkova, Y.E.; Karpenko, K.A.; Ushakov, I.E.; Goloveshkin, A.S. Direct four-component assembling of arylaldehydes, dimethylbarbituric acid, 4-hydroxycoumarine, and cyclic amines into complex scaffolds with three different heterocyclic rings. Monatsh. Chem. 2021, 152, 1327–1336. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkova, Y.E.; Karpenko, K.A.; Ushakov, I.E.; Maslov, O.I.; Egorov, M.P. Four-component transformation of benzaldehydes, dimethylbarbituric acid, 4-hydroxy-6-methyl-2H-pyran-2-one, and morpholine into the unsymmetrical ionic scaffold with three different heterocyclic rings. Russ. Chem. Bull. 2022, 71, 464–473. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, S.; Ghosh, S.K.; Ghosh, A.; Saha, R.; Banerjee, S.; Saha, B. Review of the aldol reaction. Synth. Commun. 2016, 46, 1327–1342. [Google Scholar] [CrossRef]
- Bell, R.P.; Higginson, W.C.E. The catalysed dehydration of acetaldehyde hydrate and the effect of structure on the velocity of protolytic reactions. Proc. Roy. Soc. 1949, A197, 141–159. [Google Scholar] [CrossRef]
- SciFinderⁿ—Chemical Compound Database, American Chemical Society. Available online: https://scifinder.cas.org (accessed on 10 February 2023).
- ACD/ChemSketch, version 2021.1.2; Advanced Chemistry Development, Inc. (ACD/Labs): Toronto, ON, Canada, 2021; Available online: https://www.acdlabs.com (accessed on 10 February 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N. Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate. Molbank 2023, 2023, M1589. https://doi.org/10.3390/M1589
Ryzhkova YE, Ryzhkov FV, Elinson MN. Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate. Molbank. 2023; 2023(1):M1589. https://doi.org/10.3390/M1589
Chicago/Turabian StyleRyzhkova, Yuliya E., Fedor V. Ryzhkov, and Michail N. Elinson. 2023. "Triethylammonium 2-(3-Hydroxy-2-oxoindolin-3-yl)-5,5-dimethyl-3-oxocyclohex-1-en-1-olate" Molbank 2023, no. 1: M1589. https://doi.org/10.3390/M1589