Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease
Abstract
:1. Introduction
2. Gut Microbiota
2.1. Gut-Blood Barrier (GBB) and Microbiota
2.2. Gut Microbiota-Derived Metabolites
3. Tryptophan Metabolism
3.1. Kynurenine Pathway of Tryptophan Metabolism
3.2. Serotonergic Pathway of Tryptophan Metabolism
3.3. Bacterial Metabolism of Tryptophan
3.3.1. Formation of IPA by Gut Microbiota
3.3.2. Formation of Other Indoles by Gut Microbiota
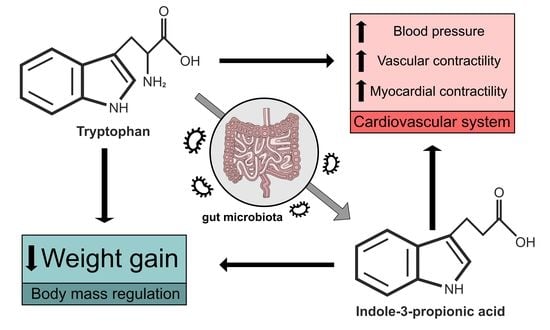
4. Biological Effects of Tryptophan and IPA
4.1. Tryptophan and Immune System
4.2. Tryptophan and Body Mass Regulation
4.3. Tryptophan and Cardiovascular System Regulation
5. Biological Effects of IPA and Its Impact on Health in Mammals
5.1. IPA Improves Gut-Blood Barrier Function
5.2. IPA Protects against Oxidative Stress and Attenuates Inflammation
5.3. IPA Protects against Carcinogens and Has an Antitumor Potential
5.4. IPA Has a Protective Role in Neurodegenerative Disease Models
5.4.1. IPA in Alzheimer’s Disease
5.4.2. IPA and Other Neurodegenerative Diseases
5.5. IPA Has a Positive Impact on Cardiovascular Disease Risk Factors
5.5.1. Diet
5.5.2. Dyslipidaemia
5.5.3. Obesity
5.5.4. Hyperglycaemia
5.5.5. Hypertension
6. Modulation of IPA Concentration as a Therapeutic Target
6.1. Antibiotics
6.2. Tryptophan Concentration in Diet
6.3. Probiotics
7. Materials and Methods
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C.D. The gut microbiome and its role in obesity. Nutr Today 2016, 51, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durgan, D.J.; Ganesh, B.P.; Cope, J.L.; Ajami, N.J.; Phillips, S.C.; Petrosino, J.F.; Hollister, E.B.; Bryan, R.M., Jr. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 2016, 67, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Kozniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 2019, 471, 1441–1453. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Ussher, J.R.; Lopaschuk, G.D.; Arduini, A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis 2013, 231, 456–461. [Google Scholar] [CrossRef]
- Li, D.Y.; Tang, W.H.W. Gut microbiota and atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 39. [Google Scholar] [CrossRef]
- Konopelski, P.; Ufnal, M. Indoles—Gut bacteria metabolites of tryptophan with pharmacotherapeutic potential. Curr. Drug Metab. 2018, 19, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Huc, T.; Konop, M.; Onyszkiewicz, M.; Podsadni, P.; Szczepanska, A.; Turlo, J.; Ufnal, M. Colonic indole, gut bacteria metabolite of tryptophan, increases portal blood pressure in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R646–R655. [Google Scholar] [CrossRef]
- Lekawanvijit, S. Role of gut-derived protein-bound uremic toxins in cardiorenal syndrome and potential treatment modalities. Circ. J. 2015, 79, 2088–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huc, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef]
- Knudsen, C.; Neyrinck, A.M.; Leyrolle, Q.; Baldin, P.; Leclercq, S.; Rodriguez, J.; Beaumont, M.; Cani, P.D.; Bindels, L.B.; Lanthier, N.; et al. Hepatoprotective effects of indole, a gut microbial metabolite, in leptin-deficient obese mice. J. Nutr. 2021, 151, 1507–1516. [Google Scholar] [CrossRef]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Renal. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Lekawanvijit, S.; Kompa, A.R.; Manabe, M.; Wang, B.H.; Langham, R.G.; Nishijima, F.; Kelly, D.J.; Krum, H. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS ONE 2012, 7, e41281. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liu, S. Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci. 2017, 185, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Melhem, N.J.; Taleb, S. Tryptophan: From diet to cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef]
- Konopelski, P.; Chabowski, D.; Aleksandrowicz, M.; Kozniewska, E.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R969–R981. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. J. Agric. Food. Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef] [PubMed]
- Garcez, M.L.; Tan, V.X.; Heng, B.; Guillemin, G.J. Sodium Butyrate and Indole-3-propionic acid prevent the increase of cytokines and kynurenine levels in LPS-induced human primary astrocytes. Int. J. Tryptophan. Res. 2020, 13, 1178646920978404. [Google Scholar] [CrossRef]
- Du, L.; Qi, R.; Wang, J.; Liu, Z.; Wu, Z. Indole-3-Propionic acid, a functional metabolite of clostridium sporogenes, promotes muscle tissue development and reduces muscle cell inflammation. Int. J. Mol. Sci. 2021, 22, 12435. [Google Scholar] [CrossRef]
- Iwan, P.; Stepniak, J.; Karbownik-Lewinska, M. Cumulative protective effect of melatonin and indole-3-propionic acid against KIO3-induced lipid peroxidation in porcine thyroid. Toxics 2021, 9, 89. [Google Scholar] [CrossRef]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.K.; Yoo, K.Y.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Jeong, Y.G.; Lee, Y.L.; Kim, Y.M.; Kwon, Y.G.; et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J. Neurosci. Res. 2009, 87, 2126–2137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mello, V.D.; Paananen, J.; Lindstrom, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamaki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef] [PubMed]
- Ruebel, M.L.; Piccolo, B.D.; Mercer, K.E.; Pack, L.; Moutos, D.; Shankar, K.; Andres, A. Obesity leads to distinct metabolomic signatures in follicular fluid of women undergoing in vitro fertilization. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E383–E396. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Ravcheev, D.; de Crecy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitek, L.; Majer, F.; Muchova, L.; Zelenka, J.; Jiraskova, A.; Branny, P.; Malina, J.; Ubik, K. Identification of bilirubin reduction products formed by Clostridium perfringens isolated from human neonatal fecal flora. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 833, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, s15–s20. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life. Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- James, K.R.; Gomes, T.; Elmentaite, R.; Kumar, N.; Gulliver, E.L.; King, H.W.; Stares, M.D.; Bareham, B.R.; Ferdinand, J.R.; Petrova, V.N.; et al. Distinct microbial and immune niches of the human colon. Nat. Immunol. 2020, 21, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, R.; Boix, J.; Odena, G.; De la Ossa, N.D.; de Vega, V.M.; Lorenzo-Zuniga, V. Colonoscopy in rats: An endoscopic, histological and tomographic study. World J. Gastrointest. Endosc. 2013, 5, 226–230. [Google Scholar] [CrossRef]
- Koruda, M.J.; Rolandelli, R.H.; Bliss, D.Z.; Hastings, J.; Rombeau, J.L.; Settle, R.G. Parenteral nutrition supplemented with short-chain fatty acids: Effect on the small-bowel mucosa in normal rats. Am. J. Clin. Nutr. 1990, 51, 685–689. [Google Scholar] [CrossRef]
- Medina, V.; Afonso, J.J.; Alvarez-Arguelles, H.; Hernandez, C.; Gonzalez, F. Sodium butyrate inhibits carcinoma development in a 1,2-dimethylhydrazine-induced rat colon cancer. JPEN J. Parenter Enteral. Nutr. 1998, 22, 14–17. [Google Scholar] [CrossRef]
- Konopelski, P.; Konop, M.; Perlejewski, K.; Bukowska-Osko, I.; Radkowski, M.; Onyszkiewicz, M.; Jaworska, K.; Mogilnicka, I.; Samborowska, E.; Ufnal, M. Genetically determined hypertensive phenotype affects gut microbiota composition, but not vice versa. J. Hypertens. 2021, 39, 1790–1799. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodriguez, R.; Godinez-Victoria, M.; Drago-Serrano, M.E. The Impact of Lactoferrin on the Growth of Intestinal Inhabitant Bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaworska, K.; Konop, M.; Bielinska, K.; Hutsch, T.; Dziekiewicz, M.; Banaszkiewicz, A.; Ufnal, M. Inflammatory bowel disease is associated with increased gut-to-blood penetration of short-chain fatty acids: A new, non-invasive marker of a functional intestinal lesion. Exp. Physiol. 2019, 104, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Huc, T.; Samborowska, E.; Dobrowolski, L.; Bielinska, K.; Gawlak, M.; Ufnal, M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE 2017, 12, e0189310. [Google Scholar] [CrossRef] [Green Version]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [Green Version]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-Oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Jaworska, K.; Bielinska, K.; Gawrys-Kopczynska, M.; Ufnal, M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: Implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc. Res. 2019, 115, 1948–1949. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Fitch, K.V.; Lo, J.; Kadar, H.; Knight, R.; Wong, K.; Abbara, S.; Gauguier, D.; Capeau, J.; Boccara, F.; et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS 2015, 29, 443–452. [Google Scholar] [CrossRef] [Green Version]
- McConnell, H.W.; Mitchell, S.C.; Smith, R.L.; Brewster, M. Trimethylaminuria associated with seizures and behavioural disturbance: A case report. Seizure 1997, 6, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Agus, A.; Clement, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Nowinski, A.; Ufnal, M. Gut bacteria-derived molecules as mediators and markers in cardiovascular diseases. The role of the gut-blood barrier. Kardiol. Pol. 2018, 76, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawrys-Kopczynska, M.; Konop, M.; Maksymiuk, K.; Kraszewska, K.; Derzsi, L.; Sozanski, K.; Holyst, R.; Pilz, M.; Samborowska, E.; Dobrowolski, L.; et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife 2020, 9, 57028. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Siebenaller, J.F. Co-evolution of proteins and solutions: Protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasova, L.; Grman, M.; Ondrias, K.; Ufnal, M. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health. Nutr. Metab. 2021, 18, 72. [Google Scholar] [CrossRef]
- Tomasova, L.; Dobrowolski, L.; Jurkowska, H.; Wrobel, M.; Huc, T.; Ondrias, K.; Ostaszewski, R.; Ufnal, M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide 2016, 60, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Hart, J.L. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertens. Res. 2015, 38, 13–20. [Google Scholar] [CrossRef]
- Zoccali, C.; Catalano, C.; Rastelli, S. Blood pressure control: Hydrogen sulfide, a new gasotransmitter, takes stage. Nephrol. Dial. Transplant. 2009, 24, 1394–1396. [Google Scholar] [CrossRef] [Green Version]
- Blachier, F.; Beaumont, M.; Kim, E. Cysteine-derived hydrogen sulfide and gut health: A matter of endogenous or bacterial origin. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 68–75. [Google Scholar] [CrossRef]
- Modis, K.; Wolanska, K.; Vozdek, R. Hydrogen sulfide in cell signaling, signal transduction, cellular bioenergetics and physiology in C. elegans. Gen. Physiol. Biophys. 2013, 32, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Al-Lahham, S.; Rezaee, F. Propionic acid counteracts the inflammation of human subcutaneous adipose tissue: A new avenue for drug development. Daru 2019, 27, 645–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Hori, D.; Flavahan, S.; Steppan, J.; Flavahan, N.A.; Berkowitz, D.E.; Pluznick, J.L. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genom. 2016, 48, 826–834. [Google Scholar] [CrossRef]
- Demigne, C.; Morand, C.; Levrat, M.A.; Besson, C.; Moundras, C.; Remesy, C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Robles-Vera, I.; Toral, M.; de la Visitacion, N.; Aguilera-Sanchez, N.; Redondo, J.M.; Duarte, J. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front. Physiol. 2020, 11, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knock, G.; Psaroudakis, D.; Abbot, S.; Aaronson, P.I. Propionate-induced relaxation in rat mesenteric arteries: A role for endothelium-derived hyperpolarising factor. J. Physiol. 2002, 538, 879–890. [Google Scholar] [CrossRef]
- Mortensen, F.V.; Nielsen, H.; Mulvany, M.J.; Hessov, I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 1990, 31, 1391–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulsmann, W.C. Coronary vasodilation by fatty acids. Basic Res. Cardiol. 1976, 71, 179–191. [Google Scholar] [CrossRef]
- Nutting, C.W.; Islam, S.; Daugirdas, J.T. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am. J. Physiol. 1991, 261, H561–H567. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Kanova, M.; Kohout, P. Tryptophan: A Unique Role in the Critically Ill. Int. J. Mol. Sci. 2021, 22, 11714. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, H.J.; Jusof, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-catabolizing enzymes-party of three. Front. Immunol. 2014, 5, 485. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. A Perspective on the safety of supplemental tryptophan based on its metabolic fates. J. Nutr. 2016, 146, 2601S–2608S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Deacon, A.C. The measurement of 5-hydroxyindoleacetic acid in urine. Ann. Clin. Biochem. 1994, 31, 215–232. [Google Scholar] [CrossRef]
- Wedin, M.; Mehta, S.; Angeras-Kraftling, J.; Wallin, G.; Daskalakis, K. The Role of Serum 5-HIAA as a Predictor of progression and an alternative to 24-h urine 5-HIAA in well-differentiated neuroendocrine neoplasms. Biology 2021, 10, 76. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C. The Gut Microbiota-Produced Indole-3-Propionic Acid Confers the antihyperlipidemic effect of mulberry-derived 1-Deoxynojirimycin. mSystems 2020, 5, e00313-20. [Google Scholar] [CrossRef] [PubMed]
- Mercer, K.E.; Yeruva, L.; Pack, L.; Graham, J.L.; Stanhope, K.L.; Chintapalli, S.V.; Wankhade, U.D.; Shankar, K.; Havel, P.J.; Adams, S.H.; et al. Xenometabolite signatures in the UC Davis type 2 diabetes mellitus rat model revealed using a metabolomics platform enriched with microbe-derived metabolites. Am. J. Physiol. Gastrointest. Liver. Physiol. 2020, 319, G157–G169. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Rosas, H.D.; Doros, G.; Bhasin, S.; Thomas, B.; Gevorkian, S.; Malarick, K.; Matson, W.; Hersch, S.M. A systems-level ‘’misunderstanding’’: The plasma metabolome in Huntington’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G.; Waller, J.M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch. Microbiol. 1976, 107, 283–288. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.J.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A Three-Ring Circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Huang, Y.S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 388. [Google Scholar] [CrossRef] [Green Version]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vecsei, L. Kynurenines in the Pathogenesis of Multiple Sclerosis: Therapeutic Perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Barth, H.; Raghuraman, S. Persistent infectious diseases say-IDO. Role of indoleamine-2,3-dioxygenase in disease pathogenesis and implications for therapy. Crit. Rev. Microbiol. 2014, 40, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Fang, M.; Yu, B. Pregnancy immune tolerance at the maternal-fetal interface. Int. Rev. Immunol. 2020, 39, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suda, T.; Asada, K.; Miwa, S.; Suzuki, M.; Fujie, M.; Furuhashi, K.; Nakamura, Y.; Inui, N.; Shirai, T.; et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin. Vaccine Immunol. 2012, 19, 436–442. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9749. [Google Scholar] [CrossRef] [Green Version]
- Munn, D.H.; Mellor, A.L. IDO in the tumor microenvironment: Inflammation, counter-regulation, and tolerance. Trends Immunol. 2016, 37, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-induced tryptophan breakdown is related with anemia, fatigue, and depression in cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [Green Version]
- Tsopmo, A.; Diehl-Jones, B.W.; Aluko, R.E.; Kitts, D.D.; Elisia, I.; Friel, J.K. Tryptophan released from mother’s milk has antioxidant properties. Pediatr. Res. 2009, 66, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitzer-Quintero, O.K.; Davalos-Marin, A.J.; Ortiz, G.G.; Meza, A.R.; Torres-Mendoza, B.M.; Robles, R.G.; Huerta, V.C.; Beas-Zarate, C. Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomed. Pharmacother. 2010, 64, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Calpena, A.C.; Clares, B. Evaluating the Oxidative Stress in Inflammation: Role of Melatonin. Int. J. Mol. Sci. 2015, 16, 16981–17004. [Google Scholar] [CrossRef] [Green Version]
- Yusufu, I.; Ding, K.; Smith, K.; Wankhade, U.D.; Sahay, B.; Patterson, G.T.; Pacholczyk, R.; Adusumilli, S.; Hamrick, M.W.; Hill, W.D.; et al. A Tryptophan-Deficient Diet Induces Gut Microbiota Dysbiosis and Increases Systemic Inflammation in Aged Mice. Int. J. Mol. Sci. 2021, 22, 5005. [Google Scholar] [CrossRef]
- Bortolato, M.; Frau, R.; Orru, M.; Collu, M.; Mereu, G.; Carta, M.; Fadda, F.; Stancampiano, R. Effects of tryptophan deficiency on prepulse inhibition of the acoustic startle in rats. Psychopharmacology 2008, 198, 191–200. [Google Scholar] [CrossRef]
- Konopelski, P.; Konop, M.; Gawrys-Kopczynska, M.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, reduces weight gain in rats. Nutrients 2019, 11, 591. [Google Scholar] [CrossRef] [Green Version]
- Franklin, M.; Bermudez, I.; Murck, H.; Singewald, N.; Gaburro, S. Sub-chronic dietary tryptophan depletion—An animal model of depression with improved face and good construct validity. J. Psychiatr. Res. 2012, 46, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ayaso, R.; Ghattas, H.; Abiad, M.; Obeid, O. Meal pattern of male rats maintained on amino acid supplemented diets: The effect of tryptophan, lysine, arginine, proline and threonine. Nutrients 2014, 6, 2509–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, S.N.; Aidney, F.; Klockars, A.; Prosser, C.; Carpenter, E.A.; Isgrove, K.; Levine, A.S.; Olszewski, P.K. Intragastric preloads of l-tryptophan reduce ingestive behavior via oxytocinergic neural mechanisms in male mice. Appetite 2018, 125, 278–286. [Google Scholar] [CrossRef]
- Ufnal, M.; Skrzypecki, J. Blood borne hormones in a cross-talk between peripheral and brain mechanisms regulating blood pressure, the role of circumventricular organs. Neuropeptides 2014, 48, 65–73. [Google Scholar] [CrossRef]
- Toropov, A.L.; Tsirkin, V.I.; Kostyaev, A.A. Combined effects of blood serum as a source of endogenous beta-adrenoceptor-sensitizing agent and its analogues histidine, tryptophan, tyrosine, mildronat, and preductal. Bull. Exp. Biol. Med. 2011, 151, 84–87. [Google Scholar] [CrossRef]
- Korotaeva, K.N.; Tsirkin, V.I.; Vyaznikov, V.A. Positive inotropic effect of tyrosine, histidine, and tryptophan in experiments on isolated human myocardium. Bull. Exp. Biol. Med. 2012, 153, 51–53. [Google Scholar] [CrossRef]
- Wolf, W.A.; Kuhn, D.M. Effects of L-tryptophan on blood pressure in normotensive and hypertensive rats. J. Pharmacol. Exp. Ther. 1984, 230, 324–329. [Google Scholar]
- Bertaccini, G.; Nobili, M.B. Effect of L-tryptophan on diuresis and 5-hydroxyindoleacetic acid excretion in the rat. Br. J. Pharmacol. Chemother. 1961, 17, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Reuther, E.; Weber, H.J.; Herken, H. Studies on sodium ion retention and antidiuretic effects after administration of L-tryptophan to rats. Naunyn. Schmiedebergs Arch. Pharmacol. 1977, 297, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Feltkamp, H.; Meurer, K.A.; Godehardt, E. Tryptophan-induced lowering of blood pressure and changes of serotonin uptake by platelets in patients with essential hypertension. Klin. Wochenschr. 1984, 62, 1115–1119. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell. Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Galligan, J.J. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol. Motil. 2018, 30, e13283. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Ding, W.; Ruan, Z.; Zhang, L. Enhanced Intestinal Barriers by Puerarin in Combination with Tryptophan. J. Agric. Food Chem. 2021, 69, 15575–15584. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Ramachandran, S.; Ganapathy, V. Cell-Surface and Nuclear Receptors in the Colon as Targets for Bacterial Metabolites and Its Relevance to Colon Health. Nutrients 2017, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Dutta, M.; Lim, J.J.; Cui, J.Y. PXR and the gut-liver axis: A recent update. Drug Metab. Dispos. 2021, 50, 000415. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Ecton, K.E.; Trikha, S.R.J.; Wrigley, S.D.; Thomas, K.N.; Battson, M.L.; Wei, Y.; Johnson, S.A.; Weir, T.L.; Gentile, C.L. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am. J. Physiol. Gastrointest. Liver. Physiol. 2020, 319, G51–G62. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol. Motil. 2018, 30, e13178. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and Dll4-notch signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Tanaka, S.; Watanabe, H.; Nakano, T.; Imafuku, T.; Kato, H.; Tokumaru, K.; Arimura, N.; Enoki, Y.; Maeda, H.; Tanaka, M.; et al. Indoxyl sulfate contributes to adipose tissue inflammation through the activation of NADPH oxidase. Toxins 2020, 12, 502. [Google Scholar] [CrossRef]
- Yisireyili, M.; Takeshita, K.; Saito, S.; Murohara, T.; Niwa, T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J. Med. Sci. 2017, 79, 477–486. [Google Scholar] [CrossRef]
- Poeggeler, B.; Pappolla, M.A.; Hardeland, R.; Rassoulpour, A.; Hodgkins, P.S.; Guidetti, P.; Schwarcz, R. Indole-3-propionate: A potent hydroxyl radical scavenger in rat brain. Brain Res. 1999, 815, 382–388. [Google Scholar] [CrossRef]
- Hardeland, R.; Zsizsik, B.K.; Poeggeler, B.; Fuhrberg, B.; Holst, S.; Coto-Montes, A. Indole-3-pyruvic and -propionic acids, kynurenic acid, and related metabolites as luminophores and free-radical scavengers. Adv. Exp. Med. Biol. 1999, 467, 389–395. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zasada, K.; Zygmunt, A.; Lewinski, A. Comparison of potential protective effects of melatonin, indole-3-propionic acid, and propylthiouracil against lipid peroxidation caused by potassium bromate in the thyroid gland. J. Cell Biochem. 2005, 95, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewinski, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Stasiak, M.; Zasada, K.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin restores the basal level of lipid peroxidation in rat tissues exposed to potassium bromate in vitro. Neuro. Endocrinol. Lett. 2010, 31, 363–369. [Google Scholar]
- Rynkowska, A.; Stepniak, J.; Karbownik-Lewinska, M. Melatonin and Indole-3-Propionic acid reduce oxidative damage to membrane lipids induced by high iron concentrations in porcine skin. Membranes 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Gitto, E.; Lewinski, A.; Reiter, R.J. Relative efficacies of indole antioxidants in reducing autoxidation and iron-induced lipid peroxidation in hamster testes. J. Cell. Biochem. 2001, 81, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Reiter, R.J.; Garcia, J.J.; Cabrera, J.; Burkhardt, S.; Osuna, C.; Lewinski, A. Indole-3-propionic acid, a melatonin-related molecule, protects hepatic microsomal membranes from iron-induced oxidative damage: Relevance to cancer reduction. J. Cell Biochem. 2001, 81, 507–513. [Google Scholar] [CrossRef]
- Qi, W.; Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Siu, A.W.; Garcia, J.J. Increased levels of oxidatively damaged DNA induced by chromium(III) and H2O2: Protection by melatonin and related molecules. J. Pineal Res. 2000, 29, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ortial, S.; Durand, G.; Poeggeler, B.; Polidori, A.; Pappolla, M.A.; Boker, J.; Hardeland, R.; Pucci, B. Fluorinated amphiphilic amino acid derivatives as antioxidant carriers: A new class of protective agents. J. Med. Chem. 2006, 49, 2812–2820. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum-Shavit, F.; Barak, V.; Saheb-Tamimi, K.; Grossowicz, N. Susceptibility of Legionella pneumophila grown extracellularly and in human monocytes to indole-3-propionic acid. Antimicrob. Agents Chemother. 1991, 35, 2526–2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossowicz, N. Phytohormones as specific inhibitors of Legionella pneumophila growth. Isr. J. Med. Sci. 1990, 26, 187–190. [Google Scholar]
- Chelala, C.A.; Margolin, P. Bactericidal photoproducts in medium containing riboflavin plus aromatic compounds and MnCl2. Can. J. Microbiol. 1983, 29, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, M.; Lindstrom, J.; Lehtonen, M.; Auriola, S.; Pihlajamaki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, S.; Govender, M.; Yap, S.H.; Kamarulzaman, A.; Rajasuriar, R.; Larsson, M. HIV-infected individuals on ART With impaired immune recovery have altered plasma metabolite profiles. Open Forum Infect. Dis. 2021, 8, ofab288. [Google Scholar] [CrossRef]
- Jeffrey, A.M.; Williams, G.M. Risk assessment of DNA-reactive carcinogens in food. Toxicol. Appl. Pharmacol. 2005, 207, 628–635. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Athreya, K.; Xavier, M.F. Antioxidants in the treatment of cancer. Nutr. Cancer 2017, 69, 1099–1104. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Reiter, R.J.; Cabrera, J.; Garcia, J.J. Comparison of the protective effect of melatonin with other antioxidants in the hamster kidney model of estradiol-induced DNA damage. Mutat. Res. 2001, 474, 87–92. [Google Scholar] [CrossRef]
- Owumi, S.E.; Adedara, I.A.; Oyelere, A.K. Indole-3-propionic acid mitigates chlorpyrifos-mediated neurotoxicity by modulating cholinergic and redox-regulatory systems, inflammatory stress, apoptotic responses and DNA damage in rats. Environ. Toxicol. Pharmacol. 2022, 89, 103786. [Google Scholar] [CrossRef]
- Karbownik, M.; Garcia, J.J.; Lewinski, A.; Reiter, R.J. Carcinogen-induced, free radical-mediated reduction in microsomal membrane fluidity: Reversal by indole-3-propionic acid. J. Bioenerg. Biomembr. 2001, 33, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Tolan, D.; Gandin, V.; Morrison, L.; El-Nahas, A.; Marzano, C.; Montagner, D.; Erxleben, A. Oxidative stress induced by Pt(IV) Pro-drugs based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position. Sci. Rep. 2016, 6, 29367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Bi, C.; Buac, D.; Fan, Y.; Zhang, X.; Zuo, J.; Zhang, P.; Zhang, N.; Dong, L.; Dou, Q.P. Organic cadmium complexes as proteasome inhibitors and apoptosis inducers in human breast cancer cells. J. Inorg. Biochem. 2013, 123, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, S.; Zaki, M.; Ahmad, M.; Afzal, M.; Srivastav, S.; Srikrishna, S.; Arjmand, F. Synthesis and crystal structure determination of copper(II)-complex: In vitro DNA and HSA binding, pBR322 plasmid cleavage, cell imaging and cytotoxic studies. Eur. J. Med. Chem. 2014, 83, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.W.; Cui, M.; Li, Y.; Dong, J.L.; Zhang, S.Q.; Zhu, C.C.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.C.; et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- van der Plas, E.; Schultz, J.L.; Nopoulos, P.C. The Neurodevelopmental Hypothesis of Huntington’s Disease. J. Huntingtons Dis. 2020, 9, 217–229. [Google Scholar] [CrossRef]
- Behl, C.; Davis, J.B.; Lesley, R.; Schubert, D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Matsubara, E.; Vidal, R.; Pacheco-Quinto, J.; Poeggeler, B.; Zagorski, M.; Sambamurti, K. Melatonin treatment enhances abeta lymphatic clearance in a transgenic mouse model of amyloidosis. Curr. Alzheimer Res. 2018, 15, 637–642. [Google Scholar] [CrossRef]
- Bendheim, P.E.; Poeggeler, B.; Neria, E.; Ziv, V.; Pappolla, M.A.; Chain, D.G. Development of indole-3-propionic acid (OXIGON) for Alzheimer′s disease. J. Mol. Neurosci. 2002, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.J.; Poeggeler, B.; Omar, R.A.; Chain, D.G.; Frangione, B.; Ghiso, J.; Pappolla, M.A. Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J. Biol. Chem. 1999, 274, 21937–21942. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, N.; Copes, N.; O′Neal-Moffitt, G.; Jin, J.; Buzzeo, R.; Mamcarz, M.; Tan, J.; Cao, C.; Olcese, J.M.; Arendash, G.W.; et al. Melatonin treatment restores mitochondrial function in Alzheimer’s mice: A mitochondrial protective role of melatonin membrane receptor signaling. J. Pineal Res. 2011, 51, 75–86. [Google Scholar] [CrossRef]
- Huang, Y.L.; Lin, C.H.; Tsai, T.H.; Huang, C.H.; Li, J.L.; Chen, L.K.; Li, C.H.; Tsai, T.F.; Wang, P.N. Discovery of a metabolic signature predisposing high risk patients with mild cognitive impairment to converting to Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 10903. [Google Scholar] [CrossRef]
- Morshedi, D.; Rezaei-Ghaleh, N.; Ebrahim-Habibi, A.; Ahmadian, S.; Nemat-Gorgani, M. Inhibition of amyloid fibrillation of lysozyme by indole derivatives--possible mechanism of action. FEBS J. 2007, 274, 6415–6425. [Google Scholar] [CrossRef] [PubMed]
- Mimori, S.; Kawada, K.; Saito, R.; Takahashi, M.; Mizoi, K.; Okuma, Y.; Hosokawa, M.; Kanzaki, T. Indole-3-propionic acid has chemical chaperone activity and suppresses endoplasmic reticulum stress-induced neuronal cell death. Biochem. Biophys. Res. Commun. 2019, 517, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mangalam, A.; Poisson, L.; Nemutlu, E.; Datta, I.; Denic, A.; Dzeja, P.; Rodriguez, M.; Rattan, R.; Giri, S. Profile of circulatory metabolites in a relapsing-remitting animal model of multiple sclerosis using global metabolomics. J. Clin. Cell. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Gaetani, L.; Boscaro, F.; Pieraccini, G.; Calabresi, P.; Romani, L.; Di Filippo, M.; Zelante, T. Host and Microbial Tryptophan metabolic profiling in multiple sclerosis. Front. Immunol. 2020, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Cavero, I.; Lefevre-Borg, F.; Roach, A.G. Effects of mianserin, desipramine and maprotiline on blood pressure responses evoked by acetylcholine, histamine and 5-hydroxytryptamine in rats. Br. J. Pharmacol. 1981, 74, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Gesper, M.; Nonnast, A.B.H.; Kumowski, N.; Stoehr, R.; Schuett, K.; Marx, N.; Kappel, B.A. Gut-derived metabolite Indole-3-Propionic acid modulates mitochondrial function in cardiomyocytes and alters cardiac function. Front. Med. 2021, 8, 648259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sawrey-Kubicek, L.; Beals, E.; Rhodes, C.H.; Houts, H.E.; Sacchi, R.; Zivkovic, A.M. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: A pilot study. Nutr. Res. 2020, 77, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Rao, Y.; Liu, R.; Deng, K.; Guan, Y.; Luo, D.; Mao, Q.; Yu, J.; Bo, T.; Fan, Z.; et al. Proteomics and metabolomics analyses reveal the full spectrum of inflammatory and lipid metabolic abnormalities in dyslipidemia. Biomed. Chromatogr. 2021, 35, e5183. [Google Scholar] [CrossRef]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2018, 68, 1552–1562. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Xia, B.; Tang, S.; Liu, L.; Xie, J.; Zhang, H. Bioregional alterations in gut microbiome contribute to the plasma metabolomic changes in pigs fed with inulin. Microorganisms 2020, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, J.; Ison, J.; Voor, M.J.; Tyagi, N. Probiotics stimulate bone formation in obese mice via histone methylations. Theranostics 2021, 11, 8605–8623. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Russo, G.; Piscitelli, P.; Giandalia, A.; Viazzi, F.; Pontremoli, R.; Fioretto, P.; De Cosmo, S. Atherogenic dyslipidemia and diabetic nephropathy. J. Nephrol. 2020, 33, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Mannisto, V.; Karja, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C.; Chen, Y.; Du, F.; Yang, Y.; Wu, G. Indole-3-propionic Acid-aggravated CCl4-induced Liver Fibrosis via the TGF-beta1/Smads Signaling Pathway. J. Clin. Transl. Hepatol. 2021, 9, 917–930. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, A.A.; Connaughton, R.M.; Lyons, C.L.; McMorrow, A.M.; Roche, H.M. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur. J. Pharmacol. 2016, 785, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, H.; Qiu, M.; Sun, W.; Wei, R.; Zheng, X.; Yang, Y.; Xin, X.; Zou, H.; Chen, T.; et al. Metabolic Signatures of Kidney Yang Deficiency Syndrome and Protective Effects of Two Herbal Extracts in Rats Using GC/TOF MS. Evid. Based. Complement. Alternat. Med. 2013, 2013, 540957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornfeldt, K.E.; Tabas, I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011, 14, 575–585. [Google Scholar] [CrossRef] [Green Version]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [Green Version]
- Ning, B.; Chen, Y.; Waqar, A.B.; Yan, H.; Shiomi, M.; Zhang, J.; Chen, Y.E.; Wang, Y.; Itabe, H.; Liang, J.; et al. Hypertension enhances advanced atherosclerosis and induces cardiac death in watanabe heritable hyperlipidemic rabbits. Am. J. Pathol. 2018, 188, 2936–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Tsai, Y.C.; Nieves, K.; Alston, L.; Mani, S.; McCoy, K.D.; Hollenberg, M.D.; Hirota, S.A. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E350–E361. [Google Scholar] [CrossRef]
- Patterson, R.A.; Stankewicz, H.A. Penicillin Allergy; Stat Pearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Zhu, D.; Sorg, J.A.; Sun, X. Clostridioides difficile biology: Sporulation, germination, and corresponding therapies for C. difficile infection. Front. Cell Infect. Microbiol. 2018, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.A.; Riviere, J.E. Comparative pharmacokinetics of aminoglycoside antibiotics. J. Vet. Pharmacol. Ther. 1991, 14, 1–35. [Google Scholar] [CrossRef]
- Behr, C.; Kamp, H.; Fabian, E.; Krennrich, G.; Mellert, W.; Peter, E.; Strauss, V.; Walk, T.; Rietjens, I.; van Ravenzwaay, B. Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats. Arch. Toxicol. 2017, 91, 3439–3454. [Google Scholar] [CrossRef]
- Wilkins, T.; Sequoia, J. Probiotics for gastrointestinal conditions: A summary of the evidence. Am. Fam. Physician 2017, 96, 170–178. [Google Scholar] [PubMed]
- Sebastian Domingo, J.J. Review of the role of probiotics in gastrointestinal diseases in adults. Gastroenterol. Hepatol. 2017, 40, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Rutten, N.B.; Besseling-van der Vaart, I.; Niers, L.E.; Choi, Y.H.; Rijkers, G.T.; van Hemert, S. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef. Microbes. 2015, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Primec, M.; Klemenak, M.; Di Gioia, D.; Aloisio, I.; Bozzi Cionci, N.; Quagliariello, A.; Gorenjak, M.; Micetic-Turk, D.; Langerholc, T. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-alpha and short-chain fatty acids. Clin. Nutr. 2019, 38, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar] [CrossRef]
- Szajewska, H.; Gyrczuk, E.; Horvath, A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: A randomized, double-blind, placebo-controlled trial. J. Pediatr. 2013, 162, 257–262. [Google Scholar] [CrossRef]



| Impact of a Factor on Cardiovascular Health | Factor Affecting Cardiovascular Health | Change in the Synthesis of IPA | References |
|---|---|---|---|
| Positive | Mediterranean diet | Increase | [168] |
| Increased composition of fibre in the diet | Increase | [29,141] | |
| Increased mulberry consumption | Increase | [83] | |
| Negative | Diabetes | Decrease | [29] |
| Dyslipidaemia | Decrease | [169] | |
| Obesity | Decrease | [30] | |
| Atherosclerosis | Decrease | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. https://doi.org/10.3390/ijms23031222
Konopelski P, Mogilnicka I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. International Journal of Molecular Sciences. 2022; 23(3):1222. https://doi.org/10.3390/ijms23031222
Chicago/Turabian StyleKonopelski, Piotr, and Izabella Mogilnicka. 2022. "Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease" International Journal of Molecular Sciences 23, no. 3: 1222. https://doi.org/10.3390/ijms23031222