Synthesis of Hybrid Epoxy Methacrylate Resin Based on Diglycidyl Ethers and Coatings Preparation via Cationic and Free-Radical Photopolymerization
Abstract
:1. Introduction
2. Results and Discussion
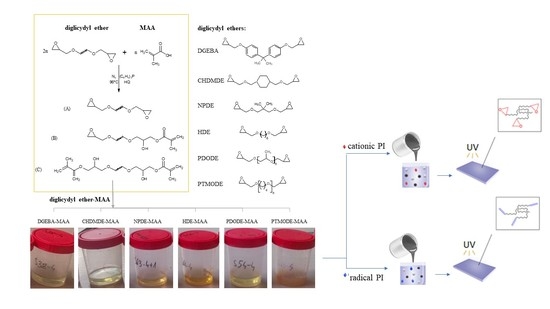
2.1. The Pre-Polymers Synthesis and Characterization
2.2. The Photocuring of the Epoxy Methacrylate Pre-Polymers
3. Materials and Methods
3.1. Materials
- −
- Bisphenol A diglycidyl ether (DGEBA), Sigma–Aldrich (Dorset, UK) with an epoxide equivalent of 171.4 g/mol and viscosity of 5032 mPa∙s at 25 °C.
- −
- Cyclohexane dimethanol diglycidyl ether (CHDMDE) under the trade name Grilonit® V 51-63, EMS-GRILTECH (Domat/Ems, Switzerland), with an epoxide equivalent of 158.8 g/mol and viscosity of 70 mPa∙s at 25 °C.
- −
- Neopentyl Glycol Diglycidyl Ether (NPDE), Tokyo Chemical Industry (Tokyo, Japan), characterized by the epoxide equivalent of 146.8 g/mol and viscosity of 10 mPa∙s at 25 °C.
- −
- 1,6-Hexanediol diglycidyl ether (HDE) under the trade name Grilonit® RV 1812, EMS-GRILTECH (Domat/Ems, Switzerland), characterized by the epoxide equivalent of 140 g/mol and viscosity of 7 mPa∙s at 25 °C.
- −
- Poly (propylene glycol) diglycidyl ether (PDODE), with a molecular weight of 380 g/mol, epoxide equivalent of 158.8 g/mol, and viscosity of 41 mPa∙s at 25 °C, Sigma–Aldrich (Dorset, UK).
- −
- Polytetrahydrofurane diglycidyl ether (PTMODE) under the trade name Grilonit® F 713, EMS-GRILTECH (Domat/Ems, Switzerland), with a molecular weight of 780 g/mol, epoxide equivalent of 400.0 g/mol, and viscosity of 230 mPa∙s at 25 °C.
3.2. Synthesis
3.3. Preparation of Coating Formulations and Cured Films
3.4. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bednarczyk, P.; Mozelewska, K.; Czech, Z. Influence of the UV crosslinking method on the properties of acrylic adhesive. Int. J. Adhes. Adhes. 2020, 102, 102652. [Google Scholar] [CrossRef]
- Gziut, K.; Kowalczyk, A.; Schmidt, B. Free-Radical Bulk-Photopolymerization Process as a Method of Obtaining Thermally Curable Structural Self-Adhesive Tapes and Effect of Used Type I Photoinitiators. Polymers 2020, 12, 2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liang, H.; Yang, H.; Xiong, L.; Zhou, J.; Huang, S.; Zhao, C.; Zhong, J.; Fan, X. UV-curable self-healing polyurethane coating based on thiol-ene and Diels-Alder double click reactions. Prog. Org. Coatings 2019, 137, 105282. [Google Scholar] [CrossRef]
- Wagner, A.; Mühlberger, M.; Paulik, C. Photoinitiator-free photopolymerization of acrylate-bismaleimide mixtures and their application for inkjet printing. J. Appl. Polym. Sci. 2019, 136, 47789. [Google Scholar] [CrossRef]
- Lorusso, E.; Ali, W.; Hildebrandt, M.; Mayer-Gall, T.; Gutmann, J.S. Hydrogel functionalized polyester fabrics by UV-induced photopolymerization. Polymers 2019, 11, 1329. [Google Scholar] [CrossRef] [Green Version]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevée, J. Radical photoinitiation with LEDs and applications in the 3D printing of composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef]
- Yagci, Y.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coatings 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Xue, X.; Yang, H. UV curable EA-Si hybrid coatings prepared by combination of radical and cationic photopolymerization. Prog. Org. Coatings 2015, 85, 46–51. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Chartrain, N.; Meenakshisundaram, V.; Feller, K.D.; Williams, C.B.; Long, T.E. 10th Anniversary: Vat Photopolymerization-Based Additive Manufacturing Current Trends and Future Directions in Materials Design. Ind. Eng. Chem. Res. 2019, 58, 15109–15118. [Google Scholar] [CrossRef]
- Glöckner, P.; Jung, T.; Struck, S.; Studer, K. Radiation Curing, Coatings and Pronting Inks, Technocal Basics, Applications and Trouble Shooting; European Coatings Tech Files; Vincentz Network: Hannover, Germany, 2008; ISBN 978-3-86630-907-4. [Google Scholar]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- UV Curable Resins & Formulated Products Market Size, Share, Forecast, (n.d.). Available online: https://www.verifiedmarketresearch.com/product/uv-curable-resins-formulated-products-market/ (accessed on 27 April 2021).
- Launikitis, M.B. Vinyl Ester Resins. In Handbook of Composites; Springer: Boston, MA, USA, 1982; pp. 38–49. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Tondi, G.; Zaske, O.C.; Goodman, S.H. Unsaturated Polyesters and Vinyl Esters. In Handbook of Thermoset Plastics; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 111–172. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. New trends in vinyl ester resins. Rev. Chem. Eng. 2014, 43, 567–581. [Google Scholar] [CrossRef]
- Scott, T.F.; Cook, W.D.; Forsythe, J.S. Photo-DSC cure kinetics of vinyl ester resins. I. Influence of temperature. Polymer 2002, 43, 5839–5845. [Google Scholar] [CrossRef]
- Park, Y.J.; Lim, D.H.; Kim, H.J.; Park, D.S.; Sung, I.K. UV- and thermal-curing behaviors of dual-curable adhesives based on epoxy acrylate oligomers. Int. J. Adhes. Adhes. 2009, 29, 710–717. [Google Scholar] [CrossRef]
- Yildiz, Z.; Gungor, A.; Onen, A.; Usta, I. Synthesis and characterization of dual-curable epoxyacrylates for polyester cord/rubber applications. J. Ind. Text. 2016, 46, 596–610. [Google Scholar] [CrossRef]
- Su, Y.C.; Cheng, L.P.; Cheng, K.C.; Don, T.M. Synthesis and characterization of UV-and thermo-curable difunctional epoxyacrylates. Mater. Chem. Phys. 2012, 132, 540–549. [Google Scholar] [CrossRef]
- Kardar, P.; Ebrahimi, M.; Bastani, S. Study the effect of nano-alumina particles on physical-mechanical properties of UV cured epoxy acrylate via nano-indentation. Prog. Org. Coatings 2008, 62, 321–325. [Google Scholar] [CrossRef]
- Biswas, K.; Gilmer, D.; Ghezawi, N.; Cao, P.F.; Saito, T. Demonstration of self-healing barrier films for vacuum insulation panels. Vacuum 2019, 164, 132–139. [Google Scholar] [CrossRef]
- EPONTM Resin 8111, (n.d.). Available online: https://www.hexion.com/en-US/product/epon-resin-8111 (accessed on 7 June 2021).
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Vidil, T.; Cloitre, M.; Tournilhac, F. Control of Gelation and Network Properties of Cationically Copolymerized Mono-and Diglycidyl Ethers. Macromolecules 2018, 51, 5121–5137. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Novel Multifunctional Epoxy (Meth)Acrylate Resins and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Polymers 2021, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Mozelewska, K.; Nowak, M.; Czech, Z. Photocurable Epoxy Acrylate Coatings Preparation by Dual Cationic and Radical Photocrosslinking. Materials 2021, 14, 4150. [Google Scholar] [CrossRef] [PubMed]
- Arbain, N.H.; Salimon, J. Synthesis And Characterization Of Ester Trimethylolpropane Based Jatropha Curcas Oil As Biolubricant Base Stocks. J. Sci. Technol. 2010, 2, 47–58. [Google Scholar]





| Sample Code | NV (%) | PAVs (mg KOH/g) | MAAC (%) | EE (g/mol) | EGC (%) | η (mPa·s) |
|---|---|---|---|---|---|---|
| DGEBA-MAA | 98.53 | 10.2 | 93.5 | 312.753 | 59.1 | 21320 |
| CHDMDE-MAA | 95.11 | 26.1 | 88.3 | 250.675 | 36.7 | 487 |
| NPDE-MAA | 88.10 | 50.2 | 85.1 | 298.973 | 50.9 | 317 |
| HDE-MAA | 96.63 | 4.2 | 97.8 | 258.150 | 45.7 | 104 |
| PDODE-MAA | 94.13 | 32.9 | 77.0 | 288.651 | 35.4 | 203 |
| PTMODE-MAA | 98.43 | 9.3 | 86.8 | 676.633 | 40.9 | 397 |
| Sample Code | n—Number of Polymer Segments | Molar Ratios of Individual Products | ||
|---|---|---|---|---|
| A | B | C | ||
| DGEBA-MAA | 1 | 0.94 | 1.00 | 0.83 |
| CHDMDE-MAA | 1 | 0.00 | 1.00 | 0.00 |
| NPDE-MAA | 1 | 0.00 | 1.00 | 1.08 |
| HDE-MAA | 1 | 1.20 | 1.00 | 1.13 |
| PDODE-MAA | 3 | 0.00 | 1.00 | 0.11 |
| PTMODE-MAA | 9 | 0.22 | 1.00 | 0.00 |
| Sample Code | ΔHtotal (J/g) | tmax (s) | pmax (%) | Rpmax (%/s) | ||||
|---|---|---|---|---|---|---|---|---|
| C | R | C | R | C | R | C | R | |
| DGEBA-MAA | 138.0 | 163.7 | 0.07 | 0.07 | 25 | 45 | 32.53 | 58.40 |
| CHDMDE-MAA | 325.4 | 400.8 | 0.26 | 0.05 | 33 | 49 | 31.79 | 65.54 |
| NPDE-MAA | 284.8 | 506 | 0.22 | 0.03 | 18 | 76 | 18.97 | 118.13 |
| HDE-MAA | 98.6 | 120.5 | 0.10 | 0.05 | 6 | 23 | 5.63 | 29.72 |
| PDODE-MAA | 107.8 | 120.3 | 0.21 | 0.20 | 4 | 10 | 5.22 | 8.95 |
| PTMODE-MAA | 23.2 | 28.0 | 0.02 | 0.01 | 3 | 4 | 4.36 | 4.38 |
| Sample Code | Tack-Free Time (s) | Hardness (s) | Adhesion | Gloss (GU) | Yellowness Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | R | C | R | C | R | C | R | C | R | |
| DGEBA-MAA | 15 | 9 | 143 | 161 | 2.5 | 2 | 165 | 160 | 4.2 | 4.1 |
| CHDMDE-MAA | 90 | 21 | 108 | 120 | 1 | 1 | 148 | 154 | 7.3 | 6.9 |
| NPDE-MAA | 90 | 90 | 66 | 67 | 2 | 2.5 | 180 | 64 | 3.9 | 3.0 |
| HDE-MAA | 90 | 90 | 54 | 63 | 1 | 2.5 | 134 | 130 | 8.3 | 7.3 |
| PDODE-MAA | 90 | 90 | 32 | 52 | 1 | 2.5 | 150 | 52 | 5.1 | 3.4 |
| PTMODE-MAA | 90 | - | 30 | - | 3.5 | - | 150 | - | 7.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Irska, I.; Gziut, K.; Mozelewska, K.; Ossowicz-Rupniewska, P. Synthesis of Hybrid Epoxy Methacrylate Resin Based on Diglycidyl Ethers and Coatings Preparation via Cationic and Free-Radical Photopolymerization. Int. J. Mol. Sci. 2022, 23, 15592. https://doi.org/10.3390/ijms232415592
Bednarczyk P, Irska I, Gziut K, Mozelewska K, Ossowicz-Rupniewska P. Synthesis of Hybrid Epoxy Methacrylate Resin Based on Diglycidyl Ethers and Coatings Preparation via Cationic and Free-Radical Photopolymerization. International Journal of Molecular Sciences. 2022; 23(24):15592. https://doi.org/10.3390/ijms232415592
Chicago/Turabian StyleBednarczyk, Paulina, Izabela Irska, Konrad Gziut, Karolina Mozelewska, and Paula Ossowicz-Rupniewska. 2022. "Synthesis of Hybrid Epoxy Methacrylate Resin Based on Diglycidyl Ethers and Coatings Preparation via Cationic and Free-Radical Photopolymerization" International Journal of Molecular Sciences 23, no. 24: 15592. https://doi.org/10.3390/ijms232415592