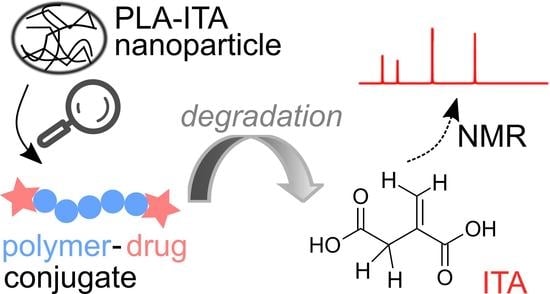
Degradation of Polymer-Drug Conjugate Nanoparticles Based on Lactic and Itaconic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of PLA-ITA Polymer-Drug Conjugate
2.2. Preparation of PLA-ITA Polymer-Drug Conjugate Nanoparticles
2.3. Identification of Degradation Products of PLA-ITA Nanoparticles
2.4. Degradation Mechanism of PLA-ITA Nanoparticles
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of the Two-Directional Polymer PLA-ITA
3.3. Preparation of PLA-ITA Nanoparticles
3.4. Degradation of PLA-ITA Nanoparticles
3.5. Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003702-1. [Google Scholar]
- WHO Tuberculosis (TB) Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 18 October 2022).
- Conde, M.B.; Efron, A.; Laredo, C.; Muzy De Souza, G.R.; Graca, N.P.; Cezar, M.C.; Ram, M.; Chaudhary, M.A.; Bishai, W.R.; Kritski, A.L.; et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: A double-blind, randomised, controlled phase II trial. Lancet 2009, 373, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Dawson, R.; Diacon, A.H.; Everitt, D.; van Niekerk, C.; Donald, P.R.; Burger, D.A.; Schall, R.; Spigelman, M.; Conradie, A.; Eisenach, K.; et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: A phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015, 385, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Boeree, M.J.; Heinrich, N.; Aarnoutse, R.; Diacon, A.H.; Dawson, R.; Rehal, S.; Kibiki, G.S.; Churchyard, G.; Sanne, I.; Ntinginya, N.E.; et al. High-Dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: A multi-arm, multi-stage randomised controlled trial. Lancet Infect. Dis. 2017, 17, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Gegia, M.; Winters, N.; Benedetti, A.; van Soolingen, D.; Menzies, D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 223–234. [Google Scholar] [CrossRef]
- Baranyai, Z.; Soria-Carrera, H.; Alleva, M.; Millan-Placer, A.C.; Lucia, A.; Martin-Rapun, R.; Ainsa, J.A.; de la Fuente, J.M. Nanotechnology-Based Targeted Drug Delivery: An Emerging Tool to Overcome Tuberculosis. Adv. Ther. 2021, 4, 2000113. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F.; Tang, R.; Wong, W.-T. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Lai, W.-F. Non-conjugated polymers with intrinsic luminescence for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101916. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Yang, L.; Wang, Z.; Wang, Z.; Feng, J.; Wen, X.; Cheng, L.; Zhu, R. MgFe-LDH Nanoparticles: A Promising Leukemia Inhibitory Factor Replacement for Self-Renewal and Pluripotency Maintenance in Cultured Mouse Embryonic Stem Cells. Adv. Sci. 2021, 8, 2003535. [Google Scholar] [CrossRef]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic Acid Is a Mammalian Metabolite Induced during Macrophage Activation. J. Am. Chem. Soc. 2011, 133, 16386–16389. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Guo, Y.; Liu, Z.; Yang, J.; Tang, H.; Wang, Y. Itaconic acid exerts anti-inflammatory and antibacterial effects via promoting pentose phosphate pathway to produce ROS. Sci. Rep. 2021, 11, 18173. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmaele, D.; Gref, R.; Couvreur, P. Squalenoylation: A generic platform for nanoparticular drug delivery. J. Control. Release 2012, 161, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, J. Paclitaxel-Initiated, Controlled Polymerization of Lactide for the Formulation of Polymeric Nanoparticulate Delivery Vehicles. Angew. Chem. Int. Ed. 2008, 47, 4830–4834. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, J. Controlled Synthesis of Camptothecin—Polylactide Conjugates and Nanoconjugates. Bioconjug. Chem. 2010, 21, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Tong, R.; Cheng, J. Drug-Initiated, Controlled Ring-Opening Polymerization for the Synthesis of Polymer—Drug Conjugates. Macromolecules 2012, 45, 2225–2232. [Google Scholar] [CrossRef]
- Yin, Q.; Tong, R.; Xu, Y.; Dobrucki, L.W.; Fan, T.M.; Cheng, J. Drug-Initiated Ring-Opening Polymerization of O-Carboxyanhydrides for the Preparation of Anticancer Drug–Poly(O-carboxyanhydride) Nanoconjugates. Biomacromolecules 2013, 14, 920–929. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J. Drug-Initiated Synthesis of Polymer Prodrugs: Combining Simplicity and Efficacy in Drug Delivery. Chem. Mater. 2016, 28, 1591–1606. [Google Scholar] [CrossRef] [Green Version]
- Pancani, E.; Menendez-Miranda, M.; Pastor, A.; Brisset, F.; Bernet-Camard, M.-F.; Desmaele, D.; Gref, R. Nanoparticles with high payloads of pipemidic acid, a poorly soluble crystalline drug: Drug-initiated polymerization and self-assembly approach. Acta Pharm. Sin. B 2018, 8, 420–431. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based nanoparticles: An overview of biomedical applications. J. Controlled Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.B.; Rohde, K.H.; Hsu, F.-F.; Russell, D.G. aprABC: A Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol. Microbiol. 2011, 80, 678–694. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Scorpio, A.; Nikaido, H.; Sun, Z.H. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 1999, 181, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- Pages, G.; Gilard, V.; Martino, R.; Malet-Martino, M. Pulsed-field gradient nuclear magnetic resonance measurements (PFG NMR) for diffusion ordered spectroscopy (DOSY) mapping. Analyst 2017, 142, 3771–3796. [Google Scholar] [CrossRef]
- Mayer, C. NMR studies of nanoparticles. Annu. Rep. Nmr Spectrosc. 2005, 55, 205–258. [Google Scholar] [CrossRef]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Phyo, P.; Zhao, X.; Templeton, A.C.; Xu, W.; Cheung, J.K.; Su, Y. Understanding molecular mechanisms of biologics drug delivery and stability from NMR spectroscopy. Adv. Drug Deliv. Rev. 2021, 174, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lamch, L.; Tylus, W.; Jewginski, M.; Latajka, R.; Wilk, K.A. Location of Varying Hydrophobicity Zinc(II) Phthalocyanine-Type Photosensitizers in Methoxy Poly(ethylene oxide) and Poly(L-lactide) Block Copolymer Micelles Using H-1 NMR and XPS Techniques. J. Phys. Chem. B 2016, 120, 12768–12780. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Torres, D.; Martin-Pastor, M.; Alonso, M.J. Application of NMR spectroscopy to the characterization of PEG-stabilized lipid nanoparticles. Langmuir 2004, 20, 8839–8845. [Google Scholar] [CrossRef]
- Heald, C.R.; Stolnik, S.; Kujawinski, K.S.; De Matteis, C.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Poly(lactic acid)-poly(ethylene oxide) (PLA-PEG) nanoparticles: NMR studies of the central solidlike PLA core and the liquid PEG corona. Langmuir 2002, 18, 3669–3675. [Google Scholar] [CrossRef]
- Okuda, T.; Ishimoto, K.; Ohara, H.; Kobayashi, S. Renewable Biobased Polymeric Materials: Facile Synthesis of Itaconic Anhydride-Based Copolymers with Poly(l-lactic acid) Grafts. Macromolecules 2012, 45, 4166–4174. [Google Scholar] [CrossRef]
- Carrasco, F.; Pages, P.; Gamez-Perez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Ural, M.S.; Menéndez-Miranda, M.; Salzano, G.; Mathurin, J.; Aybeke, E.N.; Deniset-Besseau, A.; Dazzi, A.; Porcino, M.; Martineau-Corcos, C.; Gref, R. Compartmentalized Polymeric Nanoparticles Deliver Vancomycin in a pH-Responsive Manner. Pharmaceutics 2021, 13, 1992. [Google Scholar] [CrossRef]
- Espartero, J.L.; Rashkov, I.; Li, S.M.; Manolova, N.; Vert, M. NMR Analysis of Low Molecular Weight Poly(lactic acid)s. Macromolecules 1996, 29, 3535–3539. [Google Scholar] [CrossRef]
- Comisar, C.M.; Hunter, S.E.; Walton, A.; Savage, P.E. Effect of pH on ether, ester, and carbonate hydrolysis in high-temperature water. Ind. Eng. Chem. Res. 2008, 47, 577–584. [Google Scholar] [CrossRef]
- Smith, M.B. Chapter 10—Aliphatic Substitution, Nucleophilic and Organometallic. In March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-37179-3. [Google Scholar]
- Yoo, H.S.; Oh, J.E.; Lee, K.H.; Park, T.G. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm. Res. 1999, 16, 1114–1118. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Chapter 2—Overview of Controlled Release Mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: Boston, MA, USA, 2012; pp. 19–43. ISBN 978-1-4614-0880-2. [Google Scholar]






| Species | Chemical Structure and Formula | m/z of Negative Ion [M-H] |
|---|---|---|
| LA |  C3H4O2 | 89.3 |
| ITA |  C5H6O4 | 129.1 |
| ITA-propane-2-diol |  C8H12O5 | 187.1 |
| ITA-LA |  C8H10O6 | 201.1 |
| Species | MS Peak Mode | m/z | Assignment | |
|---|---|---|---|---|
| Original PLA-ITA | [M + Na]+ (n = x + y) | 3131.89 |  | n = 39 |
| 3203.91 | n = 40 | |||
| 3275.93 | n = 41 | |||
| 3164.14 |  | n = 41 | ||
| 3308.23 | n = 43 | |||
| Degraded PLA-ITA | [M + Na]+ (n = x + y) | 1867.62 |  | n = 23 |
| 1939.64 | n = 24 | |||
| [M + K]+ | 1881.58 |  | n = 24 | |
| 1953.61 | n = 25 |
| PLA-ITA NPs Degradation | % ITA Release | % LA Release | ||
|---|---|---|---|---|
| 1 Month | 2 Months | 1 Month | 2 Months | |
| pH 7.4 | 18.4 | 17.4 | 8.7 | 8.8 |
| pH 5.3 | 7.7 | 16.0 | 2.8 | 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuong, M.D.L.; Haouas, M.; Ural, M.S.; Desmaële, D.; Martineau-Corcos, C.; Gref, R. Degradation of Polymer-Drug Conjugate Nanoparticles Based on Lactic and Itaconic Acid. Int. J. Mol. Sci. 2022, 23, 14461. https://doi.org/10.3390/ijms232214461
Vuong MDL, Haouas M, Ural MS, Desmaële D, Martineau-Corcos C, Gref R. Degradation of Polymer-Drug Conjugate Nanoparticles Based on Lactic and Itaconic Acid. International Journal of Molecular Sciences. 2022; 23(22):14461. https://doi.org/10.3390/ijms232214461
Chicago/Turabian StyleVuong, Mai Dang Le, Mohamed Haouas, Merve Seray Ural, Didier Desmaële, Charlotte Martineau-Corcos, and Ruxandra Gref. 2022. "Degradation of Polymer-Drug Conjugate Nanoparticles Based on Lactic and Itaconic Acid" International Journal of Molecular Sciences 23, no. 22: 14461. https://doi.org/10.3390/ijms232214461