An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway
Abstract
:1. Introduction
1.1. Tryptophan Metabolites
1.2. Kynurenine Metabolites as Biomarkers of Human Disease
1.3. Objective of Study
2. Methods
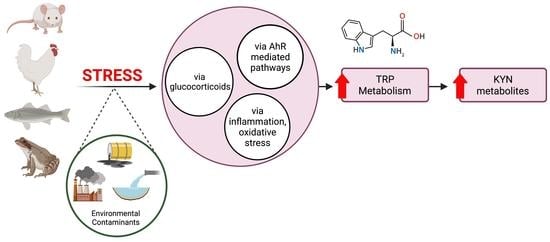
3. Discussion
3.1. Glucocorticoids and Chronic Stress
3.2. Infection, Inflammation and Oxidative Stress
3.3. Environmental Contaminants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-HAA | 3-hydroxyanthranilic acid |
| 3-HAAO | 3-hydroxyanthranilate 3,4-dioxygenase |
| 3-HK | 3-hydroxykynurenine |
| 5-HT | 5-hydroxytryptamine |
| 5-HTP | 5-hydroxytryptophan |
| AA | Anthranilic acid |
| ACMS | 2-amino-3-carboxymuconic semialdehyde |
| ACMSD | 2-amino-3-carboxymuconate semialdehyde decarboxylase |
| AhR | Aryl hydrocarbon receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ATP | Adenosine triphosphate |
| BaP | Benzo[a]pyrene |
| CAT | Catalase |
| CO2 | Carbon dioxide |
| CRH | Corticotropin releasing hormone |
| CYP | Cytochrome P450 |
| DDT | Dichlorodiphenyltrichloroethane |
| FICZ | 6-formylindolo[3,2-b]carbazole |
| GC | Glucocorticoid |
| GPx | Glutathione peroxide |
| GR | Glucocorticoid receptor |
| HPA | Hypothalamic-pituitary-adrenocortical |
| IAA | Indole-3-acetic Acid |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Proinflammatory interferon-gamma |
| IL | Interleukin |
| IPA | Indole-3-pyruvic acid |
| ITE | 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester |
| KAT | Kynurenine aminotransferase |
| KMO | Kynurenine monooxygenase |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| KYNU | Kynureninase |
| MAOA | Monoamine oxidase A |
| NAD+ | Nicotinamide adenine dinucleotide |
| NIC | Nicotinic acid |
| NMDA | N-methyl-D-aspartate |
| OS | Oxidative stress |
| PAC | Polycyclic aromatic compound |
| PAH | Polycyclic aromatic hydrocarbon |
| PIC | Picolinic acid |
| PCB | Polychlorinated biphenyl |
| PCDD | Polychlorinated dibenzo-p-dioxin |
| PCDF | Polychlorinated dibenzofuran |
| PGE3 | Prostaglandin |
| QPRT | Quinolinate phosphoribosyltransferase |
| QUIN | Quinolinic acid |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TDO | Tryptophan 2,3-dioxygenase |
| TGF-b | Tumor growth factor-beta |
| Th | T-helper |
| TNFa | Tumor necrosis factor-alpha |
| TRP | Tryptophan |
| TRP-KYN | Tryptophan-kynurenine |
| UV | Ultraviolet |
| XA | Xanthurenic acid |
References
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-033-9. [Google Scholar]
- Yin, Y. Tryptophan Metabolism in Animals Important Roles in Nutrition and Health. Front. Biosci. 2011, S3, 286–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 117864691769193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 952520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Albert, P.R.; Benkelfat, C.; Descarries, L. The Neurobiology of Depression—Revisiting the Serotonin Hypothesis. I. Cellular and Molecular Mechanisms. Phil. Trans. R. Soc. B 2012, 367, 2378–2381. [Google Scholar] [CrossRef] [Green Version]
- Oxenkrug, G.F. Tryptophan Kynurenine Metabolism as a Common Mediator of Genetic and Environmental Impacts in Major Depressive Disorder: The Serotonin Hypothesis Revisited 40 Years Later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63. [Google Scholar]
- Birkl, P.; Chow, J.; Forsythe, P.; Gostner, J.M.; Kjaer, J.B.; Kunze, W.A.; McBride, P.; Fuchs, D.; Harlander-Matauschek, A. The Role of Tryptophan-Kynurenine in Feather Pecking in Domestic Chicken Lines. Front. Vet. Sci. 2019, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The Serotonin System in Autism Spectrum Disorder: From Biomarker to Animal Models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Christoffel, D.J.; Heifets, B.D.; Ben-Dor, G.A.; Selimbeyoglu, A.; Hung, L.W.; Deisseroth, K.; Malenka, R.C. 5-HT Release in Nucleus Accumbens Rescues Social Deficits in Mouse Autism Model. Nature 2018, 560, 589–594. [Google Scholar] [CrossRef]
- Bordukalo-Niksic, T.; Cicin-Sain, L.; Jernej, B. Expression of Brain and Platelet Serotonin Transporters in Sublines of Rats with Constitutionally Altered Serotonin Homeostasis. Neurosci. Lett. 2004, 369, 44–49. [Google Scholar] [CrossRef]
- Kesić, M.; Baković, P.; Horvatiček, M.; Proust, B.L.J.; Štefulj, J.; Čičin-Šain, L. Constitutionally High Serotonin Tone Favors Obesity: Study on Rat Sublines With Altered Serotonin Homeostasis. Front. Neurosci. 2020, 14, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoseini, S.M.; Hosseini, S.A. Effect of Dietary L-Tryptophan on Osmotic Stress Tolerance in Common Carp, Cyprinus Carpio, Juveniles. Fish Physiol. Biochem. 2010, 36, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Schaeffer, H.-J. Direct Regulation of Mammalian Reproductive Organs by Serotonin and Melatonin. J. Endocrinol. 1997, 154, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Ogawa, S.; Parhar, I.S. Role of Serotonin in Fish Reproduction. Front. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The Origins of the Vertebrate Hypothalamic–Pituitary–Gonadal (HPG) and Hypothalamic–Pituitary–Thyroid (HPT) Endocrine Systems: New Insights from Lampreys. Gen. Comp. Endocrinol. 2009, 161, 20–29. [Google Scholar] [CrossRef]
- Holland, L.Z.; Albalat, R.; Azumi, K.; Benito-Gutierrez, E.; Blow, M.J.; Bronner-Fraser, M.; Brunet, F.; Butts, T.; Candiani, S.; Dishaw, L.J.; et al. The Amphioxus Genome Illuminates Vertebrate Origins and Cephalochordate Biology. Genome Res. 2008, 18, 1100–1111. [Google Scholar] [CrossRef] [Green Version]
- Kanda, S. Evolution of the Regulatory Mechanisms for the Hypothalamic-Pituitary-Gonadal Axis in Vertebrates–Hypothesis from a Comparative View. Gen. Comp. Endocrinol. 2019, 284, 113075. [Google Scholar] [CrossRef]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef]
- Namkung, Jun; Kim, Hail; Park, Sangkyu Peripheral Serotonin: A New Player in Systemic Energy Homeostasis. Mol. Cells 2015, 38, 1023–1028. [CrossRef]
- Watanabe, H.; Rose, M.; Kanayama, Y.; Shirakawa, H.; Aso, H. Energy Homeostasis by the Peripheral Serotonergic System. In Serotonin—A Chemical Messenger Between All Types of Living Cells; Shad, K.F., Ed.; IntechOpen: London, UK, 2017; ISBN 978-953-51-3361-2. [Google Scholar]
- Khan, N.; Deschaux, P. Role of Serotonin in Fish Immunomodulation. J. Exp. Biol. 1997, 200, 1833–1838. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A.-B. Tryptophan Metabolism: A Versatile Area Providing Multiple Targets for Pharmacological Intervention. Egypt. J. Basic Clin. Pharmacol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Percudani, R.; Peracchi, A. A Genomic Overview of Pyridoxal-phosphate-dependent Enzymes. EMBO Rep. 2003, 4, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.J.; Ball, H.J. Efficient Tryptophan-Catabolizing Activity Is Consistently Conserved through Evolution of TDO Enzymes, but Not IDO Enzymes. J. Exp. Zool. B. Mol. Dev. Evol. 2015, 324, 128–140. [Google Scholar] [CrossRef]
- Chen, Y.; Guillemin, G.J. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR.S2097. [Google Scholar] [CrossRef] [Green Version]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef] [Green Version]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent Evidence for an Expanded Role of the Kynurenine Pathway of Tryptophan Metabolism in Neurological Diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. An Iontophoretic Investigation of the Actions of Convulsant Kynurenines and Their Interaction with the Endogenous Excitant Quinolinic Acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of Altered NAD Metabolism in Metabolic Disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The Physiological Action of Picolinic Acid in the Human Brain. Int. J. Tryptophan Res. 2009, 2, IJTR.S2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Assessment of the Potential Role of Tryptophan as the Precursor of Serotonin and Melatonin for the Aged Sleep-Wake Cycle and Immune Function: Streptopelia Risoria as a Model. Int. J. Tryptophan Res. 2009, 2, IJTR.S1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-Enriched Cereal Intake Improves Nocturnal Sleep, Melatonin, Serotonin, and Total Antioxidant Capacity Levels and Mood in Elderly Humans. Geroscience 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangge, H.; Summers, K.L.; Meinitzer, A.; Zelzer, S.; Almer, G.; Prassl, R.; Schnedl, W.J.; Reininghaus, E.; Paulmichl, K.; Weghuber, D.; et al. Obesity-Related Dysregulation of the Tryptophan-Kynurenine Metabolism: Role of Age and Parameters of the Metabolic Syndrome: Tryptophan Metabolism and Inflammation in Obesity. Obesity 2014, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, S.; Zhong, J.; Teng, K.; Yin, Y. Crosstalk between Tryptophan Metabolism and Cardiovascular Disease, Mechanisms, and Therapeutic Implications. Oxid. Med. Cell. Longev. 2017, 2017, 1602074. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Stelzer, I.; Reininghaus, E.Z.; Weghuber, D.; Postolache, T.T.; Fuchs, D. Disturbed Tryptophan Metabolism in Cardiovascular Disease. Curr. Med. Chem. 2014, 21, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Wick, W.; Van den Eynde, B.J. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012, 72, 5435–5440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.-C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M.; et al. Gut Microbiota Dysbiosis and Altered Tryptophan Catabolism Contribute to Autoimmunity in Lupus-Susceptible Mice. Sci. Transl. Med. 2020, 12, eaax2220. [Google Scholar] [CrossRef]
- Brown, R.R.; Ozaki, Y.; Datta, S.P.; Borden, E.C.; Sondel, P.M.; Malone, D.G. Implications of Interferon-Induced Tryptophan Catabolism in Cancer, Autoimmune Diseases and Aids. In Kynurenine and Serotonin Pathways; Schwarcz, R., Young, S.N., Brown, R.R., Eds.; Advances in Experimental Medicine and Biology; Springer New York: Boston, MA, USA, 1991; Volume 294, pp. 425–435. ISBN 978-1-4684-5954-8. [Google Scholar]
- Mondanelli, G.; Iacono, A.; Carvalho, A.; Orabona, C.; Volpi, C.; Pallotta, M.T.; Matino, D.; Esposito, S.; Grohmann, U. Amino Acid Metabolism as Drug Target in Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 334–348. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How Important Is Tryptophan in Human Health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers 2022, 2022, e9484217. [Google Scholar] [CrossRef] [PubMed]
- Tan, V.X.; Guillemin, G.J. Kynurenine Pathway Metabolites as Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurosci. 2019, 13, 1013. [Google Scholar] [CrossRef] [PubMed]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic Acid Is a Potential Overlapped Biomarker between Diagnosis and Treatment Response for Depression from Metabolome Analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Modulation of Tryptophan and Serotonin Metabolism as a Biochemical Basis of the Behavioral Effects of Use and Withdrawal of Androgenic-Anabolic Steroids and Other Image- and Performance-Enhancing Agents. Int. J. Tryptophan Res. 2018, 11, 117864691775342. [Google Scholar] [CrossRef] [Green Version]
- Brooks, A.K.; Lawson, M.A.; Smith, R.A.; Janda, T.M.; Kelley, K.W.; McCusker, R.H. Interactions between Inflammatory Mediators and Corticosteroids Regulate Transcription of Genes within the Kynurenine Pathway in the Mouse Hippocampus. J. Neuroinflammation 2016, 13, 98. [Google Scholar] [CrossRef] [Green Version]
- Sorgdrager, F.J.H.; Werumeus Buning, J.; Bos, E.H.; Van Beek, A.P.; Kema, I.P. Hydrocortisone Affects Fatigue and Physical Functioning Through Metabolism of Tryptophan: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2018, 103, 3411–3419. [Google Scholar] [CrossRef]
- Lestage, J.; Verrier, D.; Palin, K.; Dantzer, R. The Enzyme Indoleamine 2,3-Dioxygenase Is Induced in the Mouse Brain in Response to Peripheral Administration of Lipopolysaccharide and Superantigen. Brain Behav. Immun. 2002, 16, 596–601. [Google Scholar] [CrossRef]
- Miura, H.; Ando, Y.; Noda, Y.; Isobe, K.; Ozaki, N. Long-Lasting Effects of Inescapable-Predator Stress on Brain Tryptophan Metabolism and the Behavior of Juvenile Mice. Stress 2011, 14, 262–272. [Google Scholar] [CrossRef]
- Mehraj, V.; Routy, J.-P. Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests. Int. J. Tryptophan Res. 2015, 8, IJTR.S26862. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 117864691985299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, T.; Sharma, S.; Kumar, M.; Hussain, M.A.; Chauhan, N.; Kalia, I.; Sahu, A.K.; Rana, V.S.; Bharti, R.; Haldar, A.K.; et al. Tryptophan-Kynurenine Pathway Attenuates β-Catenin-Dependent pro-Parasitic Role of STING-TICAM2-IRF3-IDO1 Signalosome in Toxoplasma Gondii Infection. Cell Death Dis. 2019, 10, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Q.; Hua, S.; Clish, C.B.; Scott, J.M.; Hanna, D.B.; Wang, T.; Haberlen, S.A.; Shah, S.J.; Glesby, M.J.; Lazar, J.M.; et al. Plasma Tryptophan-Kynurenine Metabolites Are Altered in Human Immunodeficiency Virus Infection and Associated With Progression of Carotid Artery Atherosclerosis. Clin. Infect. Dis. 2018, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mackay, G.M.; Forrest, C.M.; Stoy, N.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan Metabolism and Oxidative Stress in Patients with Chronic Brain Injury. Eur. J. Neurol. 2006, 13, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Petrozzi, B.; Elyamany, O.; Rummel, C.; Mulert, C. Effects of Inflammation on the Kynurenine Pathway in Schizophrenia—A Systematic Review. J. Neuroinflamm. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; Deyn, P.P.D. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10, 2565. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Cervenka, I.; Bryzgalova, G.; Dadvar, S.; Jannig, P.R.; Pettersson-Klein, A.T.; Lakshmikanth, T.; Sustarsic, E.G.; Porsmyr-Palmertz, M.; et al. Kynurenic Acid and Gpr35 Regulate Adipose Tissue Energy Homeostasis and Inflammation. Cell Metab. 2018, 27, 378–392.e5. [Google Scholar] [CrossRef] [Green Version]
- Coccaro, E.F.; Lee, R.; Fanning, J.R.; Fuchs, D.; Goiny, M.; Erhardt, S.; Christensen, K.; Brundin, L.; Coussons-Read, M. Tryptophan, Kynurenine, and Kynurenine Metabolites: Relationship to Lifetime Aggression and Inflammatory Markers in Human Subjects. Psychoneuroendocrinology 2016, 71, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Jaronen, M.; Quintana, F.J. Immunological Relevance of the Coevolution of IDO1 and AHR. Front. Immunol. 2014, 5, 521. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, J.; Wu, L.; Lu, H.; Wang, Z.; Yu, P.; Xiao, H.; Gao, R.; Yu, J. Perinatal Exposure to Bisphenol A Causes a Disturbance of Neurotransmitter Metabolic Pathways in Female Mouse Offspring: A Focus on the Tryptophan and Dopamine Pathways. Chemosphere 2020, 254, 126715. [Google Scholar] [CrossRef]
- Lin, Z.; Roede, J.R.; He, C.; Jones, D.P.; Filipov, N.M. Short-Term Oral Atrazine Exposure Alters the Plasma Metabolome of Male C57BL/6 Mice and Disrupts α-Linolenate, Tryptophan, Tyrosine and Other Major Metabolic Pathways. Toxicology 2014, 326, 130–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laffon, B.; Aguilera, F.; Ríos-Vázquez, J.; García-Lestón, J.; Fuchs, D.; Valdiglesias, V.; Pásaro, E. Endocrine and Immunological Parameters in Individuals Involved in Prestige Spill Cleanup Tasks Seven Years after the Exposure. Environ. Int. 2013, 59, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Jusof, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-Catabolizing Enzymes—Party of Three. Front. Immunol. 2014, 5, 485. [Google Scholar] [CrossRef] [Green Version]
- Nold, V.; Sweatman, C.; Karabatsiakis, A.; Böck, C.; Bretschneider, T.; Lawless, N.; Fundel-Clemens, K.; Kolassa, I.-T.; Allers, K.A. Activation of the Kynurenine Pathway and Mitochondrial Respiration to Face Allostatic Load in a Double-Hit Model of Stress. Psychoneuroendocrinology 2019, 107, 148–159. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 2470547017692328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.A.; Hightower, L.E. Stress Proteins as Molecular Biomarkers for Environmental Toxicology. In Stress-Inducible Cellular Responses; Feige, U., Yahara, I., Morimoto, R.I., Polla, B.S., Eds.; EXS; Birkhäuser: Basel, Switzerland, 1996; pp. 411–424. ISBN 978-3-0348-9088-5. [Google Scholar]
- Leonard, B.E. HPA and Immune Axes in Stress: Involvement of the Serotonergic System. Neuroimmunomodulation 2006, 13, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Isenović, E.R.; Zakula, Z.; Koricanac, G.; Ribarac-Stepić, N. Comparative Analysis of Tryptophan Oxygenase Activity and Glucocorticoid Receptor under the Influence of Insulin. Physiol. Res. 2008, 57, 101–107. [Google Scholar] [CrossRef] [PubMed]
- NIIMI, S.; NAKAMURA, T.; NAWA, K.; ICHIHARA, A. Hormonal Regulation of Translatable MRNA of Tryptophan 2,3-Dioxygenase in Primary Cultures of Adult Rat Hepatocytes1. J. Biochem. 1983, 94, 1697–1706. [Google Scholar] [CrossRef]
- Schubart, U.K. Regulation of Gene Expression in Rat Hepatocytes and Hepatoma Cells by Insulin: Quantitation of Messenger Ribonucleic Acid’s Coding for Tyrosine Aminotransferase, Tryptophan Oxygenase, and Phosphoenolpyruvate Carboxykinase. Endocrinology 1986, 119, 1741–1749. [Google Scholar] [CrossRef]
- Antunes, M.S.; Ruff, J.R.; de Oliveira Espinosa, D.; Piegas, M.B.; de Brito, M.L.O.; Rocha, K.A.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Donato, F.; et al. Neuropeptide Y Administration Reverses Tricyclic Antidepressant Treatment-Resistant Depression Induced by ACTH in Mice. Horm. Behav. 2015, 73, 56–63. [Google Scholar] [CrossRef]
- Martín-Hernández, D.; Tendilla-Beltrán, H.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C.; Caso, J.R. Chronic Mild Stress Alters Kynurenine Pathways Changing the Glutamate Neurotransmission in Frontal Cortex of Rats. Mol. Neurobiol. 2019, 56, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sheng, H.; Xu, Y.; Liu, Y.; Lu, J.; Ni, X. Swimming Exercise Ameliorates Depression-like Behavior in Chronically Stressed Rats: Relevant to Proinflammatory Cytokines and IDO Activation. Behav. Brain Res. 2013, 242, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dostal, C.R.; Carson Sulzer, M.; Kelley, K.W.; Freund, G.G.; MCusker, R.H. Glial and Tissue-Specific Regulation of Kynurenine Pathway Dioxygenases by Acute Stress of Mice. Neurobiol. Stress 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A Link between Stress and Depression: Shifts in the Balance between the Kynurenine and Serotonin Pathways of Tryptophan Metabolism and the Etiology and Pathophysiology of Depression. Stress 2008, 11, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Edelstein, M.P.; Duch, D.S. The Actions of Interferon and Antiinflammatory Agents on Induction of Indoleamine 2,3-Dioxygenase in Human Peripheral Blood Monocytes. Biochem. Biophys. Res. Commun. 1987, 144, 1147–1153. [Google Scholar] [CrossRef]
- Rose, M.; Filiatreault, A.; Guénette, J.; Williams, A.; Thomson, E.M. Ozone Increases Plasma Kynurenine-Tryptophan Ratio and Impacts Hippocampal Serotonin Receptor and Neurotrophic Factor Expression: Role of Stress Hormones. Environ. Res. 2020, 185, 109483. [Google Scholar] [CrossRef]
- Bartolini, B.; Corniello, C.; Sella, A.; Somma, F.; Politi, V. The Enol Tautomer of Indole-3-Pyruvic Acid as A Biological Switch in Stress Responses. In Developments in Tryptophan and Serotonin Metabolism; Allegri, G., Costa, C.V.L., Ragazzi, E., Steinhart, H., Varesio, L., Eds.; Advances in Experimental Medicine and Biology; Springer USA: Boston, MA, USA, 2003; pp. 601–608. ISBN 978-1-4615-0135-0. [Google Scholar]
- van Hierden, Y.M.; Koolhaas, J.M.; Korte, S.M. Chronic Increase of Dietary L-Tryptophan Decreases Gentle Feather Pecking Behaviour. Appl. Anim. Behav. Sci. 2004, 89, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Iwama, G.K. Stress in Fish. Ann. N. Y. Acad. Sci. 1998, 851, 304–310. [Google Scholar] [CrossRef]
- Herrera, M.; Fernández-Alacid, L.; Sanahuja, I.; Ibarz, A.; Salamanca, N.; Morales, E.; Giráldez, I. Physiological and Metabolic Effects of a Tryptophan-Enriched Diet to Face up Chronic Stress in Meagre (Argyrosomus regius). Aquaculture 2020, 522, 735102. [Google Scholar] [CrossRef]
- Lepage, O.; Tottmar, O.; Winberg, S. Elevated Dietary Intake of L-Tryptophan Counteracts the Stress-Induced Elevation of Plasma Cortisol in Rainbow Trout (Oncorhynchus mykiss). J. Exp. Biol. 2002, 205, 3679–3687. [Google Scholar] [CrossRef]
- Winberg, S.; Lepage, O. Elevation of Brain 5-HT Activity, POMC Expression, and Plasma Cortisol in Socially Subordinate Rainbow Trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, R645–R654. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Miró, J.M.; Giráldez, I.; Salamanca, N.; Martos-Sitcha, J.A.; Mancera, J.M.; López, J.R. Metabolic and Stress Responses in Senegalese Soles (Solea Senegalensis Kaup) Fed Tryptophan Supplements: Effects of Concentration and Feeding Period. Animals 2019, 9, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basic, D.; Krogdahl, Å.; Schjolden, J.; Winberg, S.; Vindas, M.A.; Hillestad, M.; Mayer, I.; Skjerve, E.; Höglund, E. Short- and Long-Term Effects of Dietary l-Tryptophan Supplementation on the Neuroendocrine Stress Response in Seawater-Reared Atlantic Salmon (Salmo salar). Aquaculture 2013, 388–391, 8–13. [Google Scholar] [CrossRef]
- Basic, D.; Schjolden, J.; Krogdahl, Å.; von Krogh, K.; Hillestad, M.; Winberg, S.; Mayer, I.; Skjerve, E.; Höglund, E. Changes in Regional Brain Monoaminergic Activity and Temporary Down-Regulation in Stress Response from Dietary Supplementation with l-Tryptophan in Atlantic Cod (Gadus Morhua). Br. J. Nutr. 2013, 109, 2166–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wish, J.; Bulloch, P.; Oswald, L.; Halldorson, T.; Raine, J.C.; Jamshed, L.; Marvin, C.; Thomas, P.J.; Holloway, A.C.; Tomy, G.T. Kynurenine to Tryptophan Ratio as a Biomarker of Acute Stress in Fish. Chemosphere 2022, 288, 132522. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, M.; Ueland, P.M.; Trøseid, M.; Mocroft, A.; Lebech, A.-M.; Ullum, H.; Midttun, Ø.; Lundgren, J.; Nielsen, S.D.; for the Copenhagen Comorbidity in HIV Infection (COCOMO) Study. Abdominal Adipose Tissue Is Associated With Alterations in Tryptophan-Kynurenine Metabolism and Markers of Systemic Inflammation in People With Human Immunodeficiency Virus. J. Infect. Dis. 2020, 221, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hajsl, M.; Hlavackova, A.; Broulikova, K.; Sramek, M.; Maly, M.; Dyr, J.E.; Suttnar, J. Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease. Metabolites 2020, 10, 208. [Google Scholar] [CrossRef]
- Beal, M.F.; Kowall, N.W.; Ellison, D.W.; Mazurek, M.F.; Swartz, K.J.; Martin, J.B. Replication of the Neurochemical Characteristics of Huntington’s Disease by Quinolinic Acid. Nature 1986, 321, 168–171. [Google Scholar] [CrossRef]
- Wu, W.; Nicolazzo, J.A.; Wen, L.; Chung, R.; Stankovic, R.; Bao, S.S.; Lim, C.K.; Brew, B.J.; Cullen, K.M.; Guillemin, G.J. Expression of Tryptophan 2,3-Dioxygenase and Production of Kynurenine Pathway Metabolites in Triple Transgenic Mice and Human Alzheimer’s Disease Brain. PLoS ONE 2013, 8, e59749. [Google Scholar] [CrossRef] [Green Version]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan Metabolism and Oxidative Stress in Patients with Huntington’s Disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown Is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-Associated Depression: From Serotonin to Kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.; Azeredo, R.; Domingues, A.; Fernandez-Boo, S.; Dias, J.; Conceição, L.E.C.; Costas, B. Dietary Tryptophan Deficiency and Its Supplementation Compromises Inflammatory Mechanisms and Disease Resistance in a Teleost Fish. Sci. Rep. 2019, 9, 7689. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Liu, H.; Xu, K.; Oso, A.O.; Wu, X.; Liu, G.; Tossou, M.C.B.; Al-Dhabi, N.A.; Duraipandiyan, V.; Xi, Q.; et al. A Review of the Immunomodulatory Role of Dietary Tryptophan in Livestock and Poultry. Amino Acids 2017, 49, 67–74. [Google Scholar] [CrossRef]
- Van der Leek, A.P.; Yanishevsky, Y.; Kozyrskyj, A.L. The Kynurenine Pathway As a Novel Link between Allergy and the Gut Microbiome. Front. Immunol. 2017, 8, 1374. [Google Scholar] [CrossRef] [Green Version]
- Mellor, A.L.; Munn, D.H. Tryptophan Catabolism and Regulation of Adaptive Immunity. J. Immunol. 2003, 170, 5809–5813. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.W.; Feng, G.S. Relationship between Interferon-Gamma, Indoleamine 2,3-Dioxygenase, and Tryptophan Catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Widner, B.; Ledochowski, M.; Fuchs, D. Interferon-Gamma-Induced Tryptophan Degradation: Neuropsychiatric and Immunological Consequences. Curr. Drug Metab. 2000, 1, 193–204. [Google Scholar] [CrossRef]
- Varma, T.K.; Lin, C.Y.; Toliver-Kinsky, T.E.; Sherwood, E.R. Endotoxin-Induced Gamma Interferon Production: Contributing Cell Types and Key Regulatory Factors. Clin. Diagn. Lab Immunol. 2002, 9, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.; Zhang, Z.; Nance, E.; Drewes, J.L.; Lesniak, W.G.; Singh, S.; Chugani, D.C.; Rangaramanujam, K.; Graham, D.R.; Kannan, S. Maternal Inflammation Results in Altered Tryptophan Metabolism in Rabbit Placenta and Fetal Brain. Dev. Neurosci. 2017, 39, 399–412. [Google Scholar] [CrossRef]
- Robinson, C.M.; Hale, P.T.; Carlin, J.M. The Role of IFN-Gamma and TNF-Alpha-Responsive Regulatory Elements in the Synergistic Induction of Indoleamine Dioxygenase. J. Interferon Cytokine Res. 2005, 25, 20–30. [Google Scholar] [CrossRef]
- Kwidzinski, E.; Bunse, J.; Aktas, O.; Richter, D.; Mutlu, L.; Zipp, F.; Nitsch, R.; Bechmann, I. Indolamine 2,3-Dioxygenase Is Expressed in the CNS and down-Regulates Autoimmune Inflammation. FASEB J. 2005, 19, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Liebau, C.; Merk, H.; Schmidt, S.; Roesel, C.; Karreman, C.; Prisack, J.B.; Bojar, H.; Baltzer, A.W. Interleukin-12 and Interleukin-18 Change ICAM-I Expression, and Enhance Natural Killer Cell Mediated Cytolysis of Human Osteosarcoma Cells. Cytokines Cell. Mol. Ther. 2002, 7, 135–142. [Google Scholar] [CrossRef]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2, 3-Dioxygenase Regulation of Immune Response (Review). Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T Cell Apoptosis by Tryptophan Catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Quon, T.; Lin, L.-C.; Ganguly, A.; Tobin, A.B.; Milligan, G. Therapeutic Opportunities and Challenges in Targeting the Orphan G Protein-Coupled Receptor GPR35. ACS Pharmacol. Transl. Sci. 2020, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Ligam, P.; Manuelpillai, U.; Wallace, E.M.; Walker, D. Localisation of Indoleamine 2,3-Dioxygenase and Kynurenine Hydroxylase in the Human Placenta and Decidua: Implications for Role of the Kynurenine Pathway in Pregnancy. Placenta 2005, 26, 498–504. [Google Scholar] [CrossRef]
- Sedlmayr, P.; Blaschitz, A.; Wintersteiger, R.; Semlitsch, M.; Hammer, A.; MacKenzie, C.R.; Walcher, W.; Reich, O.; Takikawa, O.; Dohr, G. Localization of Indoleamine 2,3-Dioxygenase in Human Female Reproductive Organs and the Placenta. Mol. Hum. Reprod. 2002, 8, 385–391. [Google Scholar] [CrossRef] [Green Version]
- von Rango, U.; Krusche, C.A.; Beier, H.M.; Classen-Linke, I. Indoleamine-Dioxygenase Is Expressed in Human Decidua at the Time Maternal Tolerance Is Established. J. Reprod. Immunol. 2007, 74, 34–45. [Google Scholar] [CrossRef]
- McGaha, T.L.; Huang, L.; Lemos, H.; Metz, R.; Mautino, M.; Prendergast, G.C.; Mellor, A.L. Amino Acid Catabolism: A Pivotal Regulator of Innate and Adaptive Immunity. Immunol. Rev. 2012, 249, 135–157. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The Interplay between Cytokines and the Kynurenine Pathway in Inflammation and Atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.-B.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürcü, S.; Girgin, G.; Yorulmaz, G.; Kılıçarslan, B.; Efe, B.; Baydar, T. Neopterin and Biopterin Levels and Tryptophan Degradation in Patients with Diabetes. Sci. Rep. 2020, 10, 17025. [Google Scholar] [CrossRef]
- Changsirivathanathamrong, D.; Wang, Y.; Rajbhandari, D.; Maghzal, G.J.; Mak, W.M.; Woolfe, C.; Duflou, J.; Gebski, V.; dos Remedios, C.G.; Celermajer, D.S.; et al. Tryptophan Metabolism to Kynurenine Is a Potential Novel Contributor to Hypotension in Human Sepsis. Crit. Care Med. 2011, 39, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-S.; Lee, H.-J.; Lee, D.-H.; Lee, Y.-J.; Mo, I.-P.; Nahm, S.-S.; Kim, M.-J.; Lee, J.-B.; Park, S.-Y.; Choi, I.-S.; et al. Immune Responses and Pathogenesis in Immunocompromised Chickens in Response to Infection with the H9N2 Low Pathogenic Avian Influenza Virus. Virus Res. 2008, 133, 187–194. [Google Scholar] [CrossRef]
- Yuk, S.-S.; Lee, D.-H.; Park, J.-K.; Tseren-Ochir, E.-O.; Kwon, J.-H.; Noh, J.-Y.; Lee, J.-B.; Park, S.-Y.; Choi, I.-S.; Song, C.-S. Pre-Immune State Induced by Chicken Interferon Gamma Inhibits the Replication of H1N1 Human and H9N2 Avian Influenza Viruses in Chicken Embryo Fibroblasts. Virol. J. 2016, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Santhakumar, D.; Rubbenstroth, D.; Martinez-Sobrido, L.; Munir, M. Avian Interferons and Their Antiviral Effectors. Front. Immunol. 2017, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Yang, H.; Kapczynski, D.R. Chicken Interferon Alpha Pretreatment Reduces Virus Replication of Pandemic H1N1 and H5N9 Avian Influenza Viruses in Lung Cell Cultures from Different Avian Species. Virol. J. 2011, 8, 447. [Google Scholar] [CrossRef] [Green Version]
- Emadi, M.; Jahanshiri, F.; Kaveh, K.; Hair-Bejo, M.; Ideris, A.; Alimon, R. Tryptophan Stimulates Immune Response in Broiler Chickens Challenged with Infectious Bursal Disease Vaccine. J. Anim. Vet. Adv. 2010, 9, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, DCs and Tryptophan: Much Ado about IDO. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan Metabolism, from Nutrition to Potential Therapeutic Applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Robertsen, B. The Interferon System of Teleost Fish. Fish Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. Teleost Fish Interferons and Their Role in Immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Biron, C.A.; Sen, G.C. Interferons and Other Cytokines. In Fields’ Virology; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 321–351. [Google Scholar]
- Machado, M.; Azeredo, R.; Díaz-Rosales, P.; Afonso, A.; Peres, H.; Oliva-Teles, A.; Costas, B. Dietary Tryptophan and Methionine as Modulators of European Seabass (Dicentrarchus Labrax) Immune Status and Inflammatory Response. Fish Shellfish Immunol. 2015, 42, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, S.M.; Mirghaed, A.T.; Mazandarani, M.; Zoheiri, F. Serum Cortisol, Glucose, Thyroid Hormones’ and Non-Specific Immune Responses of Persian Sturgeon, Acipenser Persicus to Exogenous Tryptophan and Acute Stress. Aquaculture 2016, 462, 17–23. [Google Scholar] [CrossRef]
- Qiu, S.; Fang, Z.; Wu, D.; Lin, Y.; Che, L. Tryptophan Supplements Promote Pregnancy Success in Mice Challenged with Pseudorabies Virus (PRV) by Regulating the Expression of Systemic Cytokines, Immunoglobulins, PRV-Specific Protein Profiles, and Toll-Like Receptors. J. Med. Food 2011, 14, 857–865. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Hougee, S.; Nap, D.; Hofenk, M.; Rombout, J.H.W.M.; van Muiswinkel, W.B.; Lidy Verburg-van Kemenade, B.M. Multiple Acute Temperature Stress Affects Leucocyte Populations and Antibody Responses in Common carp, Cyprinus carpio L. Fish Shellfish Immunol. 2003, 15, 397–410. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in Fishes: A Diversity of Responses with Particular Reference to Changes in Circulating Corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Cerqueira, M.; Schrama, D.; Silva, T.S.; Colen, R.; Engrola, S.A.D.; Conceição, L.E.C.; Rodrigues, P.M.L.; Farinha, A.P. How Tryptophan Levels in Plant-Based Aquafeeds Affect Fish Physiology, Metabolism and Proteome. J. Proteomics 2020, 221, 03782. [Google Scholar] [CrossRef]
- Kaczorek, E.; Szarek, J.; Mikiewicz, M.; Terech-Majewska, E.; Schulz, P.; Małaczewska, J.; Wójcik, R.; Siwicki, A.K. Effect of Feed Supplementation with Kynurenic Acid on the Morphology of the Liver, Kidney and Gills in Rainbow Trout (Oncorhynchus Mykiss Walbaum, 1792), Healthy and Experimentally Infected with Yersinia Ruckeri. J. Fish Dis. 2017, 40, 873–884. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, e8416763. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross Talk between ER Stress, Oxidative Stress, and Inflammation in Health and Disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [CrossRef] [PubMed]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine Pathway Metabolites and Enzymes Involved in Redox Reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef]
- Xu, K.; Liu, H.; Bai, M.; Gao, J.; Wu, X.; Yin, Y. Redox Properties of Tryptophan Metabolism and the Concept of Tryptophan Use in Pregnancy. Int. J. Mol. Sci. 2017, 18, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narïn, F.; Narin, N.; Başarslan, F.; Baykan, A.; Sezer, S.; Akgün, H.; Akin, A.; Akçakuş, M.; Ceyran, H. The Effect of L-Tryptophan on the Heart in Rabbits via Chronic Hypoxia. Turk. J. Med. Sci. 2010, 40, 257–263. [Google Scholar] [CrossRef]
- Mao, X.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.; Chen, D. The Effect of Dietary Tryptophan Levels on Oxidative Stress of Liver Induced by Diquat in Weaned Piglets. J. Anim. Sci. Biotechnol. 2014, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological Roles of Tryptophan in Teleosts: Current Knowledge and Perspectives for Future Studies. Rev. Aquac. 2019, 11, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, A. Oxidative Stress, Reactive Oxygen Species (ROS) and Antioxidative Defense System, with Special Reference to Fish. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Ji, K.; Liang, H.; Ren, M.; Ge, X.; Liu, B.; Xi, B.; Pan, L.; Yu, H. Effects of Dietary Tryptophan Levels on Antioxidant Status and Immunity for Juvenile Blunt Snout Bream (Megalobrama Amblycephala) Involved in Nrf2 and TOR Signaling Pathway. Fish Shellfish Immunol. 2019, 93, 474–483. [Google Scholar] [CrossRef]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Egerton, M.; Christofides, J.; Stone, T.W.; Darlington, L.G. Tryptophan Loading Induces Oxidative Stress. Free Radic. Res. 2004, 38, 1167–1171. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the Antioxidant Properties of Kynurenic Acid: Free Radical Scavenging Activity and Inhibition of Oxidative Stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Tryptophan-Kynurenine Pathway Is Dysregulated in Inflammation, and Immune Activation. Front. Biosci. (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Réus, G.Z.; Becker, I.R.T.; Scaini, G.; Petronilho, F.; Oses, J.P.; Kaddurah-Daouk, R.; Ceretta, L.B.; Zugno, A.I.; Dal-Pizzol, F.; Quevedo, J.; et al. The Inhibition of the Kynurenine Pathway Prevents Behavioral Disturbances and Oxidative Stress in the Brain of Adult Rats Subjected to an Animal Model of Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [Green Version]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with Neuroactive and Redox Properties: Relevance to Aging and Brain Diseases. Oxid. Med. Cell. Longev. 2014, 2014, e646909. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in Cardiovascular Diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Monosson, E. Chemical Mixtures: Considering the Evolution of Toxicology and Chemical Assessment. Environ. Health Perspect. 2005, 113, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Wallace, S.J.; de Solla, S.R.; Head, J.A.; Hodson, P.V.; Parrott, J.L.; Thomas, P.J.; Berthiaume, A.; Langlois, V.S. Polycyclic Aromatic Compounds (PACs) in the Canadian Environment: Exposure and Effects on Wildlife. Environ. Pollut. 2020, 265, 114863. [Google Scholar] [CrossRef]
- Hoshi, H.; Minamoto, N.; Iwata, H.; Shiraki, K.; Tatsukawa, R.; Tanabe, S.; Fujita, S.; Hirai, K.; Kinjo, T. Organochlorine Pesticides and Polychlorinated Biphenyl Congeners in Wild Terrestrial Mammals and Birds from Chubu Region, Japan: Interspecies Comparison of the Residue Levels and Compositions. Chemosphere 1998, 36, 3211–3221. [Google Scholar] [CrossRef]
- Lee, K.; Boufadel, M.; Chen, B.; Foght, J.; Hodson, P.; Swanson, S.; Venosa, A. Expert Panel Report on the Behaviour and Environmental Impacts of Crude Oil Released into Aqueous Environments; Royal Society of Canada: Ottawa, ON, Canada, 2015; ISBN 978-1-928140-02-3. [Google Scholar]
- Mitra, A.; Sarkar, M.; Chatterjee, C. Modulation of Immune Response by Organophosphate Pesticides: Mammals as Potential Model. Proc. Zool. Soc. 2019, 72, 13–24. [Google Scholar] [CrossRef]
- Santi, D.; Marca, A.L.; Michelangeli, M.; Casonati, A.; Grassi, R.; Baraldi, E.; Simoni, M. Ovarian Reserve and Exposure to Environmental Pollutants (ORExPo Study). In Proceedings of the Endocrine Abstracts, Bioscientifica, Bristol, UK, 1 May 2019; Volume 63. [Google Scholar]
- Tiemann, U. In Vivo and in Vitro Effects of the Organochlorine Pesticides DDT, TCPM, Methoxychlor, and Lindane on the Female Reproductive Tract of Mammals: A Review. Reprod. Toxicol. 2008, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Gutgesell, R.M.; Tsakiridis, E.E.; Jamshed, S.; Steinberg, G.R.; Holloway, A.C. Impact of Pesticide Exposure on Adipose Tissue Development and Function. Biochem J. 2020, 477, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Eng, M.L.; Stutchbury, B.J.M.; Morrissey, C.A. A Neonicotinoid Insecticide Reduces Fueling and Delays Migration in Songbirds. Science 2019, 365, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Tassin de Montaigu, C.; Goulson, D. Identifying Agricultural Pesticides That May Pose a Risk for Birds. PeerJ 2020, 8, e9526. [Google Scholar] [CrossRef]
- Murthy, K.S.; Kiran, B.; Venkateshwarlu, M. A Review on Toxicity of Pesticides in Fish. Int. J. Open Scient. Res. 2013, 1, 15–36. [Google Scholar]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A Review: Oxidative Stress in Fish Induced by Pesticides. Neuro. Endocrinol. Lett. 2009, 30 (Suppl. 1), 2–12. [Google Scholar] [PubMed]
- Ullah, S.; Zorriehzahra, J. Ecotoxicology: A Review of Pesticides Induced Toxicity in Fish. Adv. Anim. Vet. Sci. 2015, 3, 40–57. [Google Scholar] [CrossRef]
- Grosell, M.; Pasparakis, C. Physiological Responses of Fish to Oil Spills. Ann. Rev. Mar. Sci. 2021, 13, 137–160. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Cai, Z.; O’Reilly, S.E.; Hao, X.; Zhao, D. A Review of Oil, Dispersed Oil and Sediment Interactions in the Aquatic Environment: Influence on the Fate, Transport and Remediation of Oil Spills. Mar. Pollut. Bull. 2014, 79, 16–33. [Google Scholar] [CrossRef]
- Langangen, Ø.; Olsen, E.; Stige, L.C.; Ohlberger, J.; Yaragina, N.A.; Vikebø, F.B.; Bogstad, B.; Stenseth, N.C.; Hjermann, D.Ø. The Effects of Oil Spills on Marine Fish: Implications of Spatial Variation in Natural Mortality. Mar. Pollut. Bull. 2017, 119, 102–109. [Google Scholar] [CrossRef]
- Gracia, A.; Murawski, S.A.; Vázquez-Bader, A.R. Impacts of Deep Oil Spills on Fish and Fisheries. In Deep Oil Spills: Facts, Fate, and Effects; Murawski, S.A., Ainsworth, C.H., Gilbert, S., Hollander, D.J., Paris, C.B., Schlüter, M., Wetzel, D.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 414–430. ISBN 978-3-030-11605-7. [Google Scholar]
- Overturf, M.D.; Anderson, J.C.; Pandelides, Z.; Beyger, L.; Holdway, D.A. Pharmaceuticals and Personal Care Products: A Critical Review of the Impacts on Fish Reproduction. Crit. Rev. Toxicol. 2015, 45, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and Personal Care Products in Water, Sediments, Aquatic Organisms, and Fish Feeds in the Pearl River Delta: Occurrence, Distribution, Potential Sources, and Health Risk Assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute Toxicity of Pharmaceutical and Personal Care Products on Freshwater Crustacean (Thamnocephalus Platyurus) and Fish (Oryzias Latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, e2345283. [Google Scholar] [CrossRef] [Green Version]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loonen, H.; van de Guchte, C.; Parsons, J.R.; de Voogt, P.; Govers, H.A.J. Ecological Hazard Assessment of Dioxins: Hazards to Organisms at Different Levels of Aquatic Food Webs (Fish-Eating Birds and Mammals, Fish and Invertebrates). Sci. Total Environ. 1996, 182, 93–103. [Google Scholar] [CrossRef]
- Zielińska, E.; Kocki, T.; Saran, T.; Borbely, S.; Kuc, D.; Vilagi, I.; Urbańska, E.M.; Turski, W.A. Effect of Pesticides on Kynurenic Acid Production in Rat Brain Slices. Ann. Agric. Environ. Med. 2005, 12, 177–179. [Google Scholar]
- Seifert, J.; Casida, J.E. Relation of Yolk Sac Membrane Kynurenine Formamidase Inhibition to Certain Teratogenic Effects of Organophosphorus Insecticides and of Carbaryl and Eserine in Chicken Embryos. Biochem. Pharmacol. 1978, 27, 2611–2615. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Dharwadkar, S. Implications of Biotransformation of Benzo (a) Pyrene: A Review. Bionano Frontier 2011, 63–65. [Google Scholar]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novikov, O.; Wang, Z.; Stanford, E.A.; Parks, A.J.; Ramirez-Cardenas, A.; Landesman, E.; Laklouk, I.; Sarita-Reyes, C.; Gusenleitner, D.; Li, A.; et al. An Aryl Hydrocarbon Receptor-Mediated Amplification Loop That Enforces Cell Migration in ER-/PR-/Her2- Human Breast Cancer Cells. Mol. Pharmacol. 2016, 90, 674–688. [Google Scholar] [CrossRef] [Green Version]
- Rannug, A.; Rannug, U. The Tryptophan Derivative 6-Formylindolo [3,2-b]Carbazole, FICZ, a Dynamic Mediator of Endogenous Aryl Hydrocarbon Receptor Signaling, Balances Cell Growth and Differentiation. Crit. Rev. Toxicol. 2018, 48, 555–574. [Google Scholar] [CrossRef] [Green Version]
- Farmahin, R.; Crump, D.; Kennedy, S.W. Sensitivity of Avian Species to the Aryl Hydrocarbon Receptor Ligand 6-Formylindolo [3,2-b] Carbazole (FICZ). Chem. Biol. Interact. 2014, 221, 61–69. [Google Scholar] [CrossRef]
- Song, J.; Clagett-Dame, M.; Peterson, R.E.; Hahn, M.E.; Westler, W.M.; Sicinski, R.R.; DeLuca, H.F. A Ligand for the Aryl Hydrocarbon Receptor Isolated from Lung. Proc. Natl. Acad. Sci. USA 2002, 99, 14694–14699. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, P.P.; Sordillo, L.A.; Helson, L. The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res. 2017, 37, 2159–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Gil de Biedma-Elduayen, L.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the Kynurenine Pathway: A New Dancing Couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H.; Gao, X.; Jiang, J.; Xi, C.; Wu, L.; Fu, Y.; Lai, J.; Hu, S. Involvement of Kynurenine Metabolism in Bipolar Disorder: An Updated Review. Front. Psychiatry 2021, 12, 677039. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Misiak, B.; Callovini, T.; Cavaleri, D.; Cioni, R.M.; Crocamo, C.; Savitz, J.B.; Carrà, G. The Kynurenine Pathway in Bipolar Disorder: A Meta-Analysis on the Peripheral Blood Levels of Tryptophan and Related Metabolites. Mol. Psychiatry 2021, 26, 3419–3429. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The Kynurenine Pathway in Major Depressive Disorder, Bipolar Disorder, and Schizophrenia: A Meta-Analysis of 101 Studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The Kynurenine Pathway in Schizophrenia and Bipolar Disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo, E.; Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of Immune Activation on the Kynurenine Pathway and Depression Symptoms—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef]
- Brown, S.J.; Huang, X.-F.; Newell, K.A. The Kynurenine Pathway in Major Depression: What We Know and Where to Next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Serafini, G.; Adavastro, G.; Canepa, G.; Capobianco, L.; Conigliaro, C.; Pittaluga, F.; Murri, M.B.; Valchera, A.; De Berardis, D.; Pompili, M.; et al. Abnormalities in Kynurenine Pathway Metabolism in Treatment-Resistant Depression and Suicidality: A Systematic Review. CNS Neurol. Disord. Drug Targets 2017, 16, 440–453. [Google Scholar] [CrossRef]
- Cao, B.; Chen, Y.; Ren, Z.; Pan, Z.; McIntyre, R.S.; Wang, D. Dysregulation of Kynurenine Pathway and Potential Dynamic Changes of Kynurenine in Schizophrenia: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 123, 203–214. [Google Scholar] [CrossRef]
- Pukoli, D.; Polyák, H.; Rajda, C.; Vécsei, L. Kynurenines and Neurofilament Light Chain in Multiple Sclerosis. Front. Neurosci. 2021, 15, 658202. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Prabhakar, N.K.; Mannan, A. Kynurenine Metabolism and Alzheimer’s Disease: The Potential Targets and Approaches. Neurochem. Res. 2022, 47, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, D.; Iyer, M.; Narayanasamy, A.; Siva, K.; Vellingiri, B. Kynurenine Pathway in Parkinson’s Disease-an Update. Eneurologicalsci 2020, 21, 100270. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bumbu, A.G.; Andronie-Cioara, F.L.; Nechifor, A.C.; et al. The Footprint of Kynurenine Pathway in Neurodegeneration: Janus-Faced Role in Parkinson’s Disorder and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6737. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Fazio, F.; Mitsikostas, D.-D.; Martelletti, P. Fathoming the Kynurenine Pathway in Migraine: Why Understanding the Enzymatic Cascades Is Still Critically Important. Intern. Emerg. Med. 2015, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, J.; Pawlowska, E.; Szczepanska, J.; Chojnacki, C.; Blasiak, J. Kynurenine Pathway of Tryptophan Metabolism in Migraine and Functional Gastrointestinal Disorders. Int. J. Mol. Sci. 2021, 22, 10134. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Kynurenine System: Concentrations, Ratios or What Else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Dogrul, B.N. Indolamine 2,3-Dioxygenase (IDO) Inhibitors as a Potential Treatment for Somatic Symptoms. Med. Hypotheses 2022, 160, 110777. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Tóth, F.; Szabó, Á.; Vécsei, L. Co-Players in Chronic Pain: Neuroinflammation and the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 897. [Google Scholar] [CrossRef]
- Ciapała, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11055. [Google Scholar] [CrossRef]
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.; Lim, C.K.; Lovejoy, D.B.; Bessede, A.; Gluch, L.; Guillemin, G.J. Understanding the Role of the Kynurenine Pathway in Human Breast Cancer Immunobiology. Oncotarget 2016, 7, 6506–6520. [Google Scholar] [CrossRef] [Green Version]
- Kiluk, M.; Lewkowicz, J.; Pawlak, D.; Tankiewicz-Kwedlo, A. Crosstalk between Tryptophan Metabolism via Kynurenine Pathway and Carbohydrate Metabolism in the Context of Cardio-Metabolic Risk-Review. J. Clin. Med. 2021, 10, 2484. [Google Scholar] [CrossRef]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.-H. Abnormal Kynurenine Pathway of Tryptophan Catabolism in Cardiovascular Diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef]
- Gáspár, R.; Halmi, D.; Demján, V.; Berkecz, R.; Pipicz, M.; Csont, T. Kynurenine Pathway Metabolites as Potential Clinical Biomarkers in Coronary Artery Disease. Front. Immunol. 2021, 12, 768560. [Google Scholar] [CrossRef]
- Razquin, C.; Ruiz-Canela, M.; Toledo, E.; Hernández-Alonso, P.; Clish, C.B.; Guasch-Ferré, M.; Li, J.; Wittenbecher, C.; Dennis, C.; Alonso-Gómez, A.; et al. Metabolomics of the Tryptophan-Kynurenine Degradation Pathway and Risk of Atrial Fibrillation and Heart Failure: Potential Modification Effect of Mediterranean Diet. Am. J. Clin. Nutr. 2021, 114, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Melhem, N.J.; Taleb, S. Tryptophan: From Diet to Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Engin, A. The Interactions Between Kynurenine, Folate, Methionine and Pteridine Pathways in Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 511–527. ISBN 978-3-319-48382-5. [Google Scholar]
- Dadvar, S.; Ferreira, D.M.S.; Cervenka, I.; Ruas, J.L. The Weight of Nutrients: Kynurenine Metabolites in Obesity and Exercise. J. Intern. Med. 2018, 284, 519–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine Pathway Metabolism and the Microbiota-Gut-Brain Axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) Modulates Gut Homeostasis via Aryl Hydrocarbon Receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Bao, C.-H.; Wu, Y.; Liang, S.-H.; Wang, D.; Wu, L.-Y.; Huang, Y.; Liu, H.-R.; Wu, H.-G. Tryptophan-Kynurenine Metabolism: A Link between the Gut and Brain for Depression in Inflammatory Bowel Disease. J. Neuroinflamm. 2021, 18, 135. [Google Scholar] [CrossRef]
- Krupa, A.; Kowalska, I. The Kynurenine Pathway-New Linkage between Innate and Adaptive Immunity in Autoimmune Endocrinopathies. Int. J. Mol. Sci. 2021, 22, 9879. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.A.; Khan, W.I. Tryptophan-Derived Serotonin-Kynurenine Balance in Immune Activation and Intestinal Inflammation. FASEB J. 2021, 35, e21888. [Google Scholar] [CrossRef]
- Collier, M.E.; Zhang, S.; Scrutton, N.S.; Giorgini, F. Inflammation Control and Improvement of Cognitive Function in COVID-19 Infections: Is There a Role for Kynurenine 3-Monooxygenase Inhibition? Drug Discov. Today 2021, 26, 1473–1481. [Google Scholar] [CrossRef]
- Lawler, N.G.; Gray, N.; Kimhofer, T.; Boughton, B.; Gay, M.; Yang, R.; Morillon, A.-C.; Chin, S.-T.; Ryan, M.; Begum, S.; et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARS-CoV-2 Infection and Inflammatory Cytokine Responses. J. Proteome Res. 2021, 20, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Wee, H.N.; Liu, J.-J.; Ching, J.; Kovalik, J.-P.; Lim, S.C. The Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease. Am. J. Nephrol. 2021, 52, 771–787. [Google Scholar] [CrossRef]
- Mor, A.; Kalaska, B.; Pawlak, D. Kynurenine Pathway in Chronic Kidney Disease: What’s Old, What’s New, and What’s Next? Int. J. Tryptophan Res. IJTR 2020, 13, 1178646920954882. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Gilot, D.; Ahn, S.B.; Lam, V.; Shin, J.-S.; Guillemin, G.J.; Heng, B. Involvement of Kynurenine Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 5180. [Google Scholar] [CrossRef]
- Miyazaki, T.; Chung, S.; Sakai, H.; Ohata, H.; Obata, Y.; Shiokawa, D.; Mizoguchi, Y.; Kubo, T.; Ichikawa, H.; Taniguchi, H.; et al. Stemness and Immune Evasion Conferred by the TDO2-AHR Pathway Are Associated with Liver Metastasis of Colon Cancer. Cancer Sci. 2022, 113, 170–181. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The Kynurenine Connection: How Exercise Shifts Muscle Tryptophan Metabolism and Affects Energy Homeostasis, the Immune System, and the Brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Jia, L.; Quan, D.; Zhao, N.; Yang, G. Activation of the Gut Microbiota-Kynurenine-Liver Axis Contributes to the Development of Nonalcoholic Hepatic Steatosis in Nondiabetic Adults. Aging 2021, 13, 21309–21324. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.K.; Rupp, C.; Bergquist, A.; Voitl, R.; Folseraas, T.; Trøseid, M.; Midttun, Ø.; Ueland, P.M.; Karlsen, T.H.; Vesterhus, M.; et al. Associations of Neopterin and Kynurenine–Tryptophan Ratio with Survival in Primary Sclerosing Cholangitis. Scand. J. Gastroenterol. 2021, 56, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Moreau, R.; Fenaille, F.; Amorós, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [CrossRef]
- Kardashian, A.; Ma, Y.; Yin, M.T.; Scherzer, R.; Nolan, O.; Aweeka, F.; Tien, P.C.; Price, J.C. High Kynurenine:Tryptophan Ratio Is Associated With Liver Fibrosis in HIV-Monoinfected and HIV/Hepatitis C Virus–Coinfected Women. Open Forum Infect. Dis. 2019, 6, ofz281. [Google Scholar] [CrossRef]
- Ogbechi, J.; Clanchy, F.I.; Huang, Y.-S.; Topping, L.M.; Stone, T.W.; Williams, R.O. IDO Activation, Inflammation and Musculoskeletal Disease. Exp. Gerontol. 2020, 131, 110820. [Google Scholar] [CrossRef]
- Vyavahare, S.; Kumar, S.; Cantu, N.; Kolhe, R.; Bollag, W.B.; McGee-Lawrence, M.E.; Hill, W.D.; Hamrick, M.W.; Isales, C.M.; Fulzele, S. Tryptophan-Kynurenine Pathway in COVID-19-Dependent Musculoskeletal Pathology: A Minireview. Mediators Inflamm. 2021, 2021, 2911578. [Google Scholar] [CrossRef]
- Broekhuizen, M.; Danser, A.H.J.; Reiss, I.K.M.; Merkus, D. The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target? Int. J. Environ. Res. Public. Health 2021, 18, 11545. [Google Scholar] [CrossRef]
- Silvano, A.; Seravalli, V.; Strambi, N.; Cecchi, M.; Tartarotti, E.; Parenti, A.; Di Tommaso, M. Tryptophan Metabolism and Immune Regulation in the Human Placenta. J. Reprod. Immunol. 2021, 147, 103361. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Tryptophan Metabolism, Disposition and Utilization in Pregnancy. Biosci. Rep. 2015, 35, e00261. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxid. Med. Cell. Longev. 2018, 2018, 1054797. [Google Scholar] [CrossRef] [PubMed]
- Worton, S.A.; Greenwood, S.L.; Wareing, M.; Heazell, A.E.; Myers, J. The Kynurenine Pathway; A New Target for Treating Maternal Features of Preeclampsia? Placenta 2019, 84, 44–49. [Google Scholar] [CrossRef]
- Gumusoglu, S.; Scroggins, S.; Vignato, J.; Santillan, D.; Santillan, M. The Serotonin-Immune Axis in Preeclampsia. Curr. Hypertens. Rep. 2021, 23, 37. [Google Scholar] [CrossRef]
- Ala, M. The Footprint of Kynurenine Pathway in Every Cancer: A New Target for Chemotherapy. Eur. J. Pharmacol. 2021, 896, 173921. [Google Scholar] [CrossRef] [PubMed]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine Pathway, NAD+ Synthesis, and Mitochondrial Function: Targeting Tryptophan Metabolism to Promote Longevity and Healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Savitz, J. The Kynurenine Pathway: A Finger in Every Pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Badawy, A.A.-B. Kynurenine Pathway and Human Systems. Exp. Gerontol. 2020, 129, 110770. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, M.; Wu, T. Kynurenine-3-Monooxygenase: A New Direction for the Treatment in Different Diseases. Food Sci. Nutr. 2020, 8, 711–719. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int. J. Mol. Sci. 2022, 23, 6300. https://doi.org/10.3390/ijms23116300
Jamshed L, Debnath A, Jamshed S, Wish JV, Raine JC, Tomy GT, Thomas PJ, Holloway AC. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. International Journal of Molecular Sciences. 2022; 23(11):6300. https://doi.org/10.3390/ijms23116300
Chicago/Turabian StyleJamshed, Laiba, Amrita Debnath, Shanza Jamshed, Jade V. Wish, Jason C. Raine, Gregg T. Tomy, Philippe J. Thomas, and Alison C. Holloway. 2022. "An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway" International Journal of Molecular Sciences 23, no. 11: 6300. https://doi.org/10.3390/ijms23116300