Age-Dependent Contributions of NMDA Receptors and L-Type Calcium Channels to Long-Term Depression in the Piriform Cortex
Abstract
:1. Introduction
2. Results
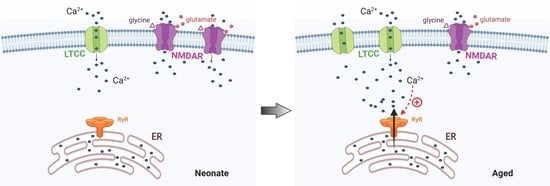
2.1. Age-Dependent Shifts in NMDAR and LTCC Contribution to LTD in Layer Ib of the Piriform Cortex
2.2. The Effects of Aging on NMDAR and LTCC Expression in the Piriform Cortex
2.3. Ryanodine Mediated Ca2+ Signalling in Aged Rats
2.4. The Contribution of GluN2B-Containining NMDARs to LTD in the Piriform Cortex
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. In Vitro Slice Preparation
4.3. Electrophysiological Field Recordings
4.4. Synaptic and Extra-Synaptic Extraction
4.5. Western Blotting
4.6. Immunohistochemistry
4.7. Confocal Imaging and Analysis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blazing, R.M.; Franks, K.M. Odor coding in piriform cortex: Mechanistic insights into distributed coding. Curr. Opin. Neurobiol. 2020, 64, 96–102. [Google Scholar] [CrossRef]
- Wilson, D.A.; Sullivan, R.M. Cortical processing of odor objects. Neuron 2011, 72, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Martin-Lopez, E.; Ishiguro, K.; Greer, C.A. The laminar organization of piriform cortex follows a selective developmental and migratory program established by cell lineage. Cerebral Cortex 2019, 29, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lebel, D.; Grossman, Y.; Barkai, E. Olfactory learning modifies predisposition for long-term potentiation and long-term depression induction in the rat piriform (olfactory) cortex. Cereb. Cortex 2001, 11, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, G.L.; Fontaine, C.J.; Harley, C.W.; Yuan, Q. A role for the anterior piriform cortex in early odor preference learning: Evidence for multiple olfactory learning structures in the rat pup. J. Neurophysiol. 2013, 110, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, B.; Yuan, Q. Nmda receptors in mouse anterior piriform cortex initialize early odor preference learning and l-type calcium channels engage for long-term memory. Sci. Rep. 2016, 6, 35256. [Google Scholar] [CrossRef] [Green Version]
- Poo, C.; Isaacson, J.S. An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex. J. Neurosci. 2007, 27, 7553–7558. [Google Scholar] [CrossRef]
- Jung, M.W.; Larson, J. Further characteristics of long-term potentiation in piriform cortex. Synapse 1994, 18, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.W.; Larson, J.; Lynch, G. Long-term potentiation of monosynaptic epsps in rat piriform cortex in vitro. Synapse 1990, 6, 279–283. [Google Scholar] [CrossRef]
- Kanter, E.D.; Haberly, L.B. Nmda-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res. 1990, 525, 175–179. [Google Scholar] [CrossRef]
- Franks, K.M.; Isaacson, J.S. Synapse-specific downregulation of nmda receptors by early experience: A critical period for plasticity of sensory input to olfactory cortex. Neuron 2005, 47, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Mukherjee, B.; Chen, X.; Yuan, Q. Beta-adrenoceptor activation enhances l-type calcium channel currents in anterior piriform cortex pyramidal cells of neonatal mice: Implication for odor learning. Learn. Mem. 2017, 24, 132–135. [Google Scholar] [CrossRef]
- Moyer, J.R., Jr.; Thompson, L.T.; Black, J.P.; Disterhoft, J.F. Nimodipine increases excitability of rabbit ca1 pyramidal neurons in an age- and concentration-dependent manner. J. Neurophysiol. 1992, 68, 2100–2109. [Google Scholar] [CrossRef]
- Moyer, J.R., Jr.; Disterhoft, J.F. Nimodipine decreases calcium action potentials in rabbit hippocampal ca1 neurons in an age-dependent and concentration-dependent manner. Hippocampus 1994, 4, 11–17. [Google Scholar] [CrossRef]
- Power, J.M.; Wu, W.W.; Sametsky, E.; Oh, M.M.; Disterhoft, J.F. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal ca1 pyramidal neurons in vitro. J. Neurosci. 2002, 22, 7234–7243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disterhoft, J.F.; Wu, W.W.; Ohno, M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and alzheimer’s disease. Ageing Res. Rev. 2004, 3, 383–406. [Google Scholar] [CrossRef]
- Disterhoft, J.F.; Oh, M.M. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell 2007, 6, 327–336. [Google Scholar] [CrossRef]
- Boric, K.; Munoz, P.; Gallagher, M.; Kirkwood, A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J. Neurosci. 2008, 28, 8034–8039. [Google Scholar] [CrossRef]
- Lee, H.K.; Min, S.S.; Gallagher, M.; Kirkwood, A. Nmda receptor-independent long-term depression correlates with successful aging in rats. Nat. Neurosci. 2005, 8, 1657–1659. [Google Scholar] [CrossRef]
- Shankar, S.; Teyler, T.J.; Robbins, N. Aging differentially alters forms of long-term potentiation in rat hippocampal area ca1. J. Neurophysiol. 1998, 79, 334–341. [Google Scholar] [CrossRef]
- Navakkode, S.; Liu, C.; Soong, T.W. Altered function of neuronal l-type calcium channels in ageing and neuroinflammation: Implications in age-related synaptic dysfunction and cognitive decline. Ageing Res. Rev. 2018, 42, 86–99. [Google Scholar] [CrossRef]
- Sidhu, V.K.; Huang, B.X.; Desai, A.; Kevala, K.; Kim, H.Y. Role of dha in aging-related changes in mouse brain synaptic plasma membrane proteome. Neurobiol. Aging 2016, 41, 73–85. [Google Scholar] [CrossRef] [Green Version]
- VanGuilder, H.D.; Yan, H.; Farley, J.A.; Sonntag, W.E.; Freeman, W.M. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J. Neurochem. 2010, 113, 1577–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierra, N.C.; Kirmiz, M.; Van Der List, D.; Santana, L.F.; Trimmer, J.S. Kv2.1 mediates spatial and functional coupling of l-type calcium channels and ryanodine receptors in mammalian neurons. eLife 2019, 8, e49953. [Google Scholar] [CrossRef]
- Dumas, T.C. Developmental regulation of cognitive abilities: Modified composition of a molecular switch turns on associative learning. Prog. Neurobiol. 2005, 76, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. Nmda receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Quinlan, E.M.; Lebel, D.; Brosh, I.; Barkai, E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron 2004, 41, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.A.; Rao, G.; Houston, F.P. Ltp induction threshold change in old rats at the perforant path—Granule cell synapse. Neurobiol. Aging 2000, 21, 613–620. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Barnes, C.A. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003, 69, 143–179. [Google Scholar] [CrossRef]
- Kumar, A.; Foster, T.C. Interaction of dhpg-ltd and synaptic-ltd at senescent ca3-ca1 hippocampal synapses. Hippocampus 2014, 24, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Norris, C.M.; Halpain, S.; Foster, T.C. Reversal of age-related alterations in synaptic plasticity by blockade of l-type Ca2+ channels. J. Neurosci. 1998, 18, 3171–3179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, C.M.; Kerr, D.S.; Abraham, W.C. Glucocorticoid receptor activation lowers the threshold for nmda-receptor-dependent homosynaptic long-term depression in the hippocampus through activation of voltage-dependent calcium channels. J. Neurophysiol. 1997, 78, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, T.C.; Norris, C.M. Age-associated changes in ca(2+)-dependent processes: Relation to hippocampal synaptic plasticity. Hippocampus 1997, 7, 602–612. [Google Scholar] [CrossRef]
- Gaburjakova, M.; Gaburjakova, J.; Reiken, S.; Huang, F.; Marx, S.O.; Rosemblit, N.; Marks, A.R. Fkbp12 binding modulates ryanodine receptor channel gating. J. Biol. Chem. 2001, 276, 16931–16935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gant, J.C.; Chen, K.C.; Kadish, I.; Blalock, E.M.; Thibault, O.; Porter, N.M.; Landfield, P.W. Reversal of aging-related neuronal Ca2+ dysregulation and cognitive impairment by delivery of a transgene encoding fk506-binding protein 12.6/1b to the hippocampus. J. Neurosci. 2015, 35, 10878–10887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gant, J.C. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 2006, 26, 3482–3490. [Google Scholar] [CrossRef] [Green Version]
- Gant, J.C.; Blalock, E.M.; Chen, K.-C.; Kadish, I.; Porter, N.M.; Norris, C.M.; Thibault, O.; Landfield, P.W. Fk506-binding protein 1b/12.6: A key to aging-related hippocampal Ca2+ dysregulation? Eur. J. Pharmacol. 2014, 739, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Hall, D.D.; Hell, J.W. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 2009, 89, 411–452. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, S.A.; Grochova, D.; McKenna, T.; Borate, B.; Trivedi, N.S.; Erdos, M.R.; Eriksson, M. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell 2016, 15, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.Z.; Liang, M.C.; Lu, S.; Yu, D.; Yu, C.Y.; Yue, D.T.; Soong, T.W. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, cav1.2 α1 subunit. J. Biol. Chem. 2004, 279, 44335–44343. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.Z.; Hong, X.; Wang, J.; Soong, T.W. Signature combinatorial splicing profiles of rat cardiac- and smooth-muscle cav1.2 channels with distinct biophysical properties. Cell Calcium 2007, 41, 417–428. [Google Scholar] [CrossRef]
- Hu, Z.; Liang, M.C.; Soong, T.W. Alternative splicing of l-type cav1.2 calcium channels: Implications in cardiovascular diseases. Genes 2017, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, A.; Ramachandran, B.; Ahmed, S.; Benito, E.; Shinoda, Y.; Nitzan, N.; Heukamp, A.; Rannio, S.; Martens, H.; Barth, J.; et al. Synaptotagmin-3 drives ampa receptor endocytosis, depression of synapse strength, and forgetting. Science 2019, 363, eaav1483. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, R.E.; Alarcon, J.M.; Malleret, G.; Carroll, R.C.; Grody, M.; Vronskaya, S.; Kandel, E.R. Transgenic mice lacking nmdar-dependent ltd exhibit deficits in behavioral flexibility. Neuron 2008, 58, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Dong, Z.; Bagot, R.C.; Howland, J.G.; Phillips, A.G.; Wong, T.P.; Wang, Y.T. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc. Natl. Acad. Sci. USA 2010, 107, 16697–16702. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, S.; Scott, H.; Glover, C.; Bienemann, A.; Ghorbel, M.T.; Uney, J.; Brown, M.W.; Warburton, E.C.; Bashir, Z.I. Expression of long-term depression underlies visual recognition memory. Neuron 2008, 58, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Turrigiano, G.G.; Nelson, S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004, 5, 97–107. [Google Scholar] [CrossRef]
- Manahan-Vaughan, D.; Braunewell, K.H. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl. Acad. Sci. USA 1999, 96, 8739–8744. [Google Scholar] [CrossRef] [Green Version]
- Best, A.R.; Wilson, D.A. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J. Neurosci. 2004, 24, 652–660. [Google Scholar] [CrossRef] [Green Version]
- Best, A.R.; Thompson, J.V.; Fletcher, M.L.; Wilson, D.A. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J. Neurosci. 2005, 25, 2513–2517. [Google Scholar] [CrossRef] [Green Version]
- Cotman, C.W.; Matthews, D.A. Synaptic plasma membranes from rat brain synaptosomes: Isolation and partial characterization. Biochim. Biophys. Acta 1971, 249, 380–394. [Google Scholar] [CrossRef]
- Nunez-Santana, F.L.; Oh, M.M.; Antion, M.D.; Lee, A.; Hell, J.W.; Disterhoft, J.F. Surface l-type ca2+ channel expression levels are increased in aged hippocampus. Aging Cell 2014, 13, 111–120. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajani, V.; Maziar, A.; Man, K.N.M.; Hell, J.W.; Yuan, Q. Age-Dependent Contributions of NMDA Receptors and L-Type Calcium Channels to Long-Term Depression in the Piriform Cortex. Int. J. Mol. Sci. 2021, 22, 13551. https://doi.org/10.3390/ijms222413551
Rajani V, Maziar A, Man KNM, Hell JW, Yuan Q. Age-Dependent Contributions of NMDA Receptors and L-Type Calcium Channels to Long-Term Depression in the Piriform Cortex. International Journal of Molecular Sciences. 2021; 22(24):13551. https://doi.org/10.3390/ijms222413551
Chicago/Turabian StyleRajani, Vishaal, Aida Maziar, Kwun Nok Mimi Man, Johannes W. Hell, and Qi Yuan. 2021. "Age-Dependent Contributions of NMDA Receptors and L-Type Calcium Channels to Long-Term Depression in the Piriform Cortex" International Journal of Molecular Sciences 22, no. 24: 13551. https://doi.org/10.3390/ijms222413551