Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes
Abstract
:1. Introduction
2. Results
2.1. Carnosic Acid Suppresses Early Increase in Superoxide Anion and ROS Levels during Adipocyte Differentiation
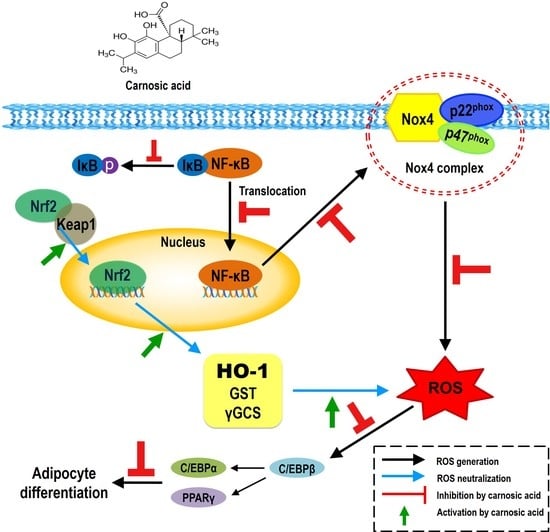
2.2. Carnosic Acid Attenuates the Translation of Nox4, p47 phox, and p22phox and Nuclear Transport of NF-κB by Inhibiting IκBα Phosphorylation
2.3. Carnosic Acid Inhibits Adipocyte Differentiation by Attenuating Nox4-Mediated ROS Generation via Interruption of NF-κB/IκBα Signal Pathway
2.4. Carnosic Acid induces Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Mediated Translation of Phase II Antioxidant Enzymes
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Cell Culture and Differentiation
4.3. Determination of Intracellular Superoxide Anion and ROS
4.4. Western Blot Analysis
4.5. Nuclear Protein Extraction
4.6. Confocal Microscopy
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DHE | Dihyroethidium |
| DCFH-DA | 2′-7′-dichlorofluorescin diacetate |
| Nox4 | NADPH (nicotinamide adenine dinucleotide phosphate) oxidase 4 |
| NF-κB | Nuclear factor-kappa B |
| IκBα | NF-κB inhibitor |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| DMEM | Dulbecco`s modified Eagle`s medium |
| MDI | Methyl-isobuthylxanthine, dexamethasone, and insulin |
| HBSS | Hank`s balanced salt solution |
| PMSF | Phenylmethanesulfonyl fluoride |
| DMSO | Dimethylsulfoxide |
| RIPA | Radio-immunoprecipitation assay |
| BAY | Bay11-7082 |
| DPAI | 4′,6-diamidine-2-phenylindole |
| HO-1 | Heme oxygenase-1 |
| γ-GCSc | γ–Glutamylcysteine synthetase |
| GST | Glutathione S-transferase |
| SPSS | Statistical Package for Social Science |
References
- Wang, Q.A.; Scherer, P.E.; Gupta, R.E. Improved methodologies formthe study of adipose biology: Insights gained and opportunities ahead. J. Lipid Res. 2014, 55, 605–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The biology of white adipocyte proliferation. Obes. Rev. 2001, 2, 239–254. [Google Scholar] [CrossRef]
- Wang, Y.W.; Jones, P.J. Conjugated linoleic acid and obesity control: Efficacy and mechanisms. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 941–955. [Google Scholar] [CrossRef] [Green Version]
- Ji, E.; Jung, M.Y.; Park, J.H.; Seo, C.R.; Park, K.W.; Lee, E.K.; Yeom, C.H.; Lee, S. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after dowmregulation of hyaluronic acid. Int. J. Obes. 2014, 38, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, D.; Srinivasan, H.; Patil, M.S.; Seetarama, S.; Kumar, S.A.; Dixit, M.N. Novel method to differentiate 3T3 L1 cells in vitro to produce highly sensitive adipocytes for a GLUT4 mediated glucose uptake using fluorescent glucose analog. J. Cell Commun. Signal. 2013, 7, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, M. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hai, C. Redox modification of adipocyte differentiation: Hypothesis of “Redox Chain” and novel insights into intervention of adipogenesis and obesity. Free Radic. Biol. Med. 2015, 89, 99–125. [Google Scholar] [CrossRef]
- Kanda, Y.; Hinata, T.; Kang, S.W.; Watanabe, Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011, 89, 250–258. [Google Scholar] [CrossRef]
- Liu, G.S.; Chan, E.C.; Higuchi, M.; Dusting, G.J.; Jiang, F. Redox mechanisms in regulation of adipocyte differentiation: Beyond a general stress response. Cells 2012, 1, 976–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Lee, H.M.; Lee, Y.J.; Choi, H.J.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [Green Version]
- Benard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar]
- Schöder, K.; Wandzioch, K.; Helmeke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arteroscler. Thromb. Vasc. Biol. 2008, 2, 239–245. [Google Scholar]
- Birtic, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activity of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Maheswarappa, N.B.; Subbaiah, V.; Muthupalani, M.; Yamagani, P.K.; Mohan, K.; Keshapaga, U.R.; Asokan, S.V.; Kalappurakkal, R.C. Antioxidant activity of carnosic acid and rosmarinic acid in raw and cooked ground chicken patties. J. Sci. Food Agric. 2014, 94, 273–279. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radical Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Yu, Y.M.; Lin, C.H.; Chan, H.C.; Tsai, H.D. Carnosic acid reduce cytokine-induced adhesion molecules expression and monocyte adhesion to endothelial cells. Eur. J. Nutr. 2009, 48, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, K.W.; Chae, I.G.; Kundu, J.; Kim, E.H.; Kundu, J.K.; Chen, K.S. Carnosic acid inhibits STAT3 signaling and induces apoptosis through generation of ROS in human colon cancer HCT116 cells. Mol. Carcinog. 2016, 55, 1096–1100. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Rhummuri, D.; Naidu, V.G.M.; Chaudhari, P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-κB and MAPK signaling. J. Mol. Med. 2017, 95, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Basnet, R.; Wiyasihati, S.; Nakata, K.; Hagiwara, K.; Miyazaki, H.; Yoshida, K. Carnosic acid inhibits the formation of osteoclasts through attenuation of expression of RANKL. PharmaNutrion 2015, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Park, M.Y.; Sung, M.K. Carnosic acid inhibits lipid accumulation in 3T3-L1 adipocytes and through attenuation of fatty acid desaturation. J. Cancer Prev. 2015, 20, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as major bioactive component in rosemary extract ameliorates high-fat-induced obesity and metabolic syndrome. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef]
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenetic effect of carnosic acid, a natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim. Biophys. Acta 2013, 1831, 3796–3806. [Google Scholar] [CrossRef]
- Takahashi, T.; Tabuchi, T.; Tamaki, Y.; Kosaka, K.; Takikawa, Y.; Sato, T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase2 enzymes and activation of glutathione metabolism. Biochem. Biophys. Res. Commun. 2009, 382, 549–554. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Wang, B.B.; Jang, H.D. Anti-osteoclastic effect of caffeic acid phenethyl ester in murine macrophages depends upon the suppression of superoxide anion production through the prevention of can active-Nox1 complex formation. J. Nutr. Biochem. 2018, 58, 158–168. [Google Scholar] [CrossRef]
- Cho, K.J.; Moon, H.E.; Moini, M.; Pecker, L.; Yoon, D.Y.; Chung, A.S. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J. Biol. Chem. 2003, 278, 34823–34833. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.T.; Chang, H.H.; Hsiao, C.H.; Lee, M.J.; Ku, H.C.; Hu, Y.J.; Kao, Y.H. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depends on the glutathione and 67 kDa laminin receptor pathways. Mol. Nutr. Food Res. 2009, 53, 349–360. [Google Scholar] [CrossRef]
- Seo, M.J.; Choi, H.S.; Lee, O.H.; Lee, B.Y. Granteloupia lanceolata (Okamura) Kawaguchi, the edible red seaweed, inhibits lipid accumulation and reactive oxygen species production during differentiation in 3T3-L1 cells. Phytother. Res. 2013, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, K.J.; Park, B.R.; Yoon, J.H.; Lim, O.H.; Lee, O.H. Buckwheat (Fago-pyrum esculetum M.) sprout treated with methyl jasmonate (MeJA) improved anti-adipogenic activity associated with the oxidative stress system in 3T3-L1 adipocyte. Int. J. Mol. Sci. 2013, 14, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Abe, D.; Saito, Y.; Kubo, Y.; Nakamura, Y.; Sekiya, K. A fraction of unripe kiwi fruit extract regulates adipocyte differentiation and function in 3T3-L1 cells. Biofactors 2010, 36, 52–59. [Google Scholar] [CrossRef]
- Martyn, K.D.; Frederick, L.M.; Loehneysen, K.V.; Dinauer, M.C.; Knaus, U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 2006, 18, 69–82. [Google Scholar] [CrossRef]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen year later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Ishi, T.; Ishikawa, M.; Miyoshi, N.; Yasunaga, M.; Akagawa, M.; Uchida, K.; Nakamura, Y. Catechol type polyphenol is a potential modifier of protein sulfhydryls: Development and application of a new probe for understanding dietary polyphenol actions. Chem. Res. Toxicol. 2009, 22, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Wang, X.J. Induction of the Keap1/Nrf2/ARE pathway by oxidizable diphenols. Chem. Biol. Interact. 2011, 192, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; McKercher, S.; Lipton, S. Nrf2/ARE-mediated antioxidant actions of pro-electric drugs. Free Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, G.; Lindroos-Christensen, J.; Einwallner, E.; Husa, J.; Zapf, T.; Lipp, K.; Rauscher, S.; Gröger, M.; Spittler, A.; Loewe, R.; et al. HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt2. Sci. Rep. 2017, 7, 40881. [Google Scholar] [CrossRef]
- Koh, E.J.; Kim, K.J.; Seo, Y.J.; Choi, J.; Lee, B.Y. Modulation of HO-1 by ferulic acid attenuates adipocyte differentiation in 3T3-L1 cells. Molecules 2017, 22, 745. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Burgess, A.; Li, M.; Tsenovoy, P.L.; Addabo, F.; McClung, J.A.; Puri, N.; Abraham, N.G. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokine tumor necrosis-α and interleuin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Phamacol. Exp. Ther. 2008, 325, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Vanella, L.; Sodhi, K.; Kim, D.H.; Puri, N.; Maheshwan, M.; Hinds, T.D.; Bellner, L.; Goldstein, D.; Peterson, S.; Shapiro, J.I.; et al. Increased heme-oxygenase-1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res. Ther. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.I.; Ko, H.C.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Lee, N.H.; Kim, S.J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, S.H.; Kim, S.Z.; Park, W.H. Caspase inhibitor decreases apoptosis in pyrogallol-treated lung cancer Calu-6 cells via the prevention of GSH depletion. Int. J. Oncol. 2008, 33, 1099–1105. [Google Scholar] [PubMed]
- Daniel, J.; Macphee, D.J. Methodological considerations for improving Western blot analysis. J. Pharmacol. Toxicol. Methods 2010, 2, 171–177. [Google Scholar]
- Grindel, S.J.; Rohe, B.; Safford, S.E.; Bennett, J.J.; Farach-Carson, M.C. Tumor necrosis factor-α treatment of HepG2 cells mobilizes a cytoplasmic pool of Erp57/1.25D3-MARRS to the nucleus. J. Cell Biochem. 2011, 112, 2606–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-K.; Jang, H.-D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int. J. Mol. Sci. 2021, 22, 6096. https://doi.org/10.3390/ijms22116096
Lee D-K, Jang H-D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. International Journal of Molecular Sciences. 2021; 22(11):6096. https://doi.org/10.3390/ijms22116096
Chicago/Turabian StyleLee, Dae-Kun, and Hae-Dong Jang. 2021. "Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes" International Journal of Molecular Sciences 22, no. 11: 6096. https://doi.org/10.3390/ijms22116096