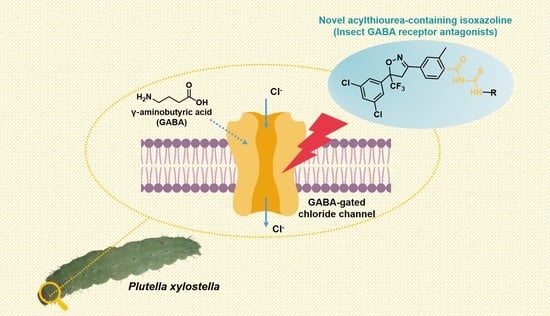
Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Compounds
2.2. Insecticidal Activity
2.3. 3D-QSAR Analysis
2.4. Design and Synthesis of Compound 32
2.5. Enzyme Activity Determination
2.6. Docking
2.7. Proteomics Analysis
2.7.1. GO Analysis
2.7.2. KEGG Classification Analysis
3. Experimental
3.1. Instruments and Chemicals
3.2. Synthesis
3.2.1. Synthesis of Intermediate B
3.2.2. Synthesis of Intermediate C
3.2.3. Preparation of Intermediates D
3.2.4. Preparation of Target Compounds 1–32
3.3. Insecticidal Activity Test
3.4. 3D-QSAR Models
3.5. Enzyme Activity Assays
3.6. Molecular Docking
3.7. Proteomics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sarfraz, M.; Keddie, A.B.; Dosdall, L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Technol. 2005, 15, 763–789. [Google Scholar] [CrossRef] [Green Version]
- Gavilanez, R.E.; Grilli, M.P. First record of the spatio-temporal variation of Plutella xylostella and its parasitoids complex in central Argentina. Biol. Control. 2019, 133, 1–8. [Google Scholar] [CrossRef]
- Newman, K.; You, M.; Vasseur, L. Diamondback moth (Lepidoptera: Plutellidae) exhibits oviposition and larval feeding pref-erences among crops, wild plants, and ornamentals as host plants. J. Econ. Entomol. 2016, 2, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, R. Historical changes in the lethal effects of insecticides against the diamondbackmoth, Plutella xylostella (L.). Pest Manag. Sci. 2021, 77, 3116–3125. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, X.; Liu, S.-S.; You, M.; Furlong, M.J. Biology, Ecology, and Management of the Diamondback Moth in China. Annu. Rev. Èntomol. 2016, 61, 277–296. [Google Scholar] [CrossRef]
- Banazeer, A.; Afzal, M.B.S.; Hassan, S.; Ijaz, M.; Shad, S.A.; Serrão, J.E. Status of insecticide resistance in Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae) from 1997 to 2019: Cross-resistance, genetics, biological costs, underlying mechanisms, and implications for management. Phytoparasitica 2021, 50, 465–485. [Google Scholar] [CrossRef]
- Shakeel, M.; Farooq, M.; Nasim, W.; Akram, W.; Khan, F.; Jaleel, W.; Zhu, X.; Yin, H.; Li, S.; Fahad, S.; et al. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: A review. Environ. Sci. Pollut. Res. 2017, 24, 14537–14550. [Google Scholar] [CrossRef]
- Zhou, J.L.; Guo, Z.J.; Kang, S.; Qin, J.Y.; Gong, L.J.; Sun, D.; Guo, L.; Zhu, L.H.; Bai, Y.; Zhang, Z.Z.; et al. Reduced expression of the P-glycoprotein gene PxABCB1 is linked to resistance to Bacillus thuringiensis Cry1Ac toxin in Plutella xylostella (L.). Pest Manag. Sci. 2019, 76, 712–720. [Google Scholar] [CrossRef]
- Shoop, W.L.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Ihara, M.; Sattelle, D.B.; Matsuda, K. Mechanisms of Action, Resistance and Toxicity of Insecticides Targeting GABA Receptors. Curr. Med. Chem. 2017, 24, 2935–2945. [Google Scholar] [CrossRef]
- García-Reynaga, P.; Zhao, C.; Sarpong, R.; Casida, J.E. New GABA/Glutamate Receptor Target for [3H]Isoxazoline Insecticide. Chem. Res. Toxicol. 2013, 26, 514–516. [Google Scholar] [CrossRef] [Green Version]
- Casida, J.E.; Durkin, K.A. Novel GABA receptor pesticide targets. Pestic. Biochem. Physiol. 2015, 121, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Rufener, L.; Danelli, V.; Bertrand, D.; Sager, H. The novel isoxazoline ectoparasiticide lotilaner (Credelio™): A non-competitive antagonist specific to invertebrates γ-aminobutyric acid-gated chloride channels (GABACls). Parasites Vectors 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Gonçalves, I.L.; das Neves, G.M.; Kagami, L.P.; Eifler-Lima, V.L.; Merlo, A.A. Discovery, development, chemical diversity and design of isoxazoline-based insecticides. Bioorganic Med. Chem. 2020, 30, 115934. [Google Scholar] [CrossRef]
- Weber, T.; Selzer, P.M. Isoxazolines: A Novel Chemotype Highly Effective on Ectoparasites. ChemMedChem 2016, 11, 270–276. [Google Scholar] [CrossRef]
- Casida, J.E. Golden Age of RyR and GABA-R Diamide and Isoxazoline Insecticides: Common Genesis, Serendipity, Surprises, Selectivity, and Safety. Chem. Res. Toxicol. 2015, 28, 560–566. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [Google Scholar] [CrossRef]
- Asahi, M.; Kobayashi, M.; Kagami, T.; Nakahira, K.; Furukawa, Y.; Ozoe, Y. Fluxametamide: A novel isoxazoline insecticide that acts via distinctive antagonism of insect ligand-gated chloride channels. Pestic. Biochem. Physiol. 2018, 151, 67–72. [Google Scholar] [CrossRef]
- Blythe, J.; Earley, F.G.; Piekarska-Hack, K.; Firth, L.; Bristow, J.; Hirst, E.A.; Goodchild, J.A.; Hillesheim, E.; Crossthwaite, A.J. The mode of action of isocycloseram: A novel isoxazoline insecticide. Pestic. Biochem. Physiol. 2022, 187, 105217. [Google Scholar] [CrossRef]
- Gassel, M.; Wolf, C.; Noack, S.; Williams, H.; Ilg, T. The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod γ-aminobutyric acid- and l-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 2014, 45, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Curtis, M.P.; Chubb, N.; Ellsworth, E.; Goodwin, R.; Holzmer, S.; Koch, J.; McTier, T.; Menon, S.; Mills, K.; Pullins, A.; et al. The discovery of isoxazoline oxime ethers as a new class of ectoparasiticides for the control of Haematobia irritans (horn fly) in cattle. Bioorganic Med. Chem. Lett. 2014, 24, 5011–5014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Plattner, J.J.; Easom, E.E.; Akama, T.; Zhou, Y.; White, W.H.; Defauw, J.M.; Winkle, J.R.; Balko, T.W.; Cao, J.; et al. Optimization of isoxazoline amide benzoxaboroles for identification of a development candidate as an oral long acting animal ectoparasiticide. Bioorganic Med. Chem. Lett. 2016, 26, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Cordova, D.; Barry, J.D.; Pahutski, T.F.; Smith, B.K.; Long, J.K.; Benner, E.A.; Holyoke, C.W.; Joraski, K.; Xu, M.; et al. 4-Azolylphenyl isoxazoline insecticides acting at the GABA gated chloride channel. Bioorganic Med. Chem. Lett. 2013, 23, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-K.; Plattner, J.J.; Easom, E.E.; Zhou, Y.; Akama, T.; Bu, W.; White, W.H.; Defauw, J.M.; Winkle, J.R.; Balko, T.W.; et al. Discovery of an orally bioavailable isoxazoline benzoxaborole (AN8030) as a long acting animal ectoparasiticide. Bioorganic Med. Chem. Lett. 2015, 25, 5589–5593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, Y.; Zheng, C.; Bai, P.; Yang, J.; Lu, X. Design, Synthesis, and Insecticidal Activity of Novel Doramectin Derivatives Containing Acylurea and Acylthiourea Based on Hydrogen Bonding. J. Agric. Food Chem. 2020, 68, 5806–5815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-F.; Xu, J.-Y.; Wang, B.-L.; Li, Y.-X.; Xiong, L.-X.; Li, Y.-Q.; Ma, Y.; Li, Z.-M. Synthesis and Insecticidal Activities of Novel Anthranilic Diamides Containing Acylthiourea and Acylurea. J. Agric. Food Chem. 2012, 60, 7565–7572. [Google Scholar] [CrossRef]
- Carmen, L.; Luminita, M.; Mariana, C.C. Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives. Molecules 2011, 16, 7593–7607. [Google Scholar]
- Saeed, A.; Batool, M. Synthesis and bioactivity of some new 1-tolyl-3-aryl-4-methylimidazole-2-thiones. Med. Chem. Res. 2007, 16, 143–154. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, Y.; Li, J.H.; Li, R.H.; Wang, J.; Li, S.X.; Gao, X.Y.; Dong, L.; Li, A.Q. Design, synthesis, herbicidal activity, in vivo enzyme activity evaluation and molecular docking study of acylthiourea derivatives as novel acetohydroxyacid synthase in-hibitor. J. Mol. Struct. 2021, 1241, 130627. [Google Scholar] [CrossRef]
- Han, J.; Dong, H.; Xu, Z.; Wang, J.; Wang, M. Synthesis and Activity of Novel Acylthiourea with Hydantoin. Int. J. Mol. Sci. 2013, 14, 19526–19539. [Google Scholar] [CrossRef]
- Hallur, G.; Jimeno, A.; Dalrymple, S.; Zhu, T.; Jung, M.K.; Hidalgo, M.; Isaacs, J.T.; Sukumar, S.; Hamel, E.; Khan, S.R. Ben-zoylphenylurea sulfur analogues with potent antitumor activity. J. Med. Chem. 2006, 49, 2357–2360. [Google Scholar] [CrossRef]
- Rao, X.-P.; Wu, Y.; Song, Z.-Q.; Shang, S.-B.; Wang, Z.-D. Synthesis and antitumor activities of unsymmetrically disubstituted acylthioureas fused with hydrophenanthrene structure. Med. Chem. Res. 2010, 20, 333–338. [Google Scholar] [CrossRef]
- Brazhe, N.A.; Nikelshparg, E.I.; Baizhumanov, A.A.; Grivennikova, V.G.; Semenova, A.A.; Novikov, S.M.; Volkov, V.S.; Arsenin, A.V.; Yakubovsky, D.I.; Evlyukhin, A.B.; et al. SERS uncovers the link between conformation of cytochrome c heme and mitochondrial membrane potential. Free Radic. Biol. Med. 2023, 196, 133–144. [Google Scholar] [CrossRef]
- Chertkova, R.V.; Brazhe, N.A.; Bryantseva, T.V.; Nekrasov, A.N.; Dolgikh, D.A.; Yusipovich, A.I.; Sosnovtseva, O.; Maksimov, G.V.; Rubin, A.B.; Kirpichnikov, M.P. New insight into the mechanism of mitochondrial cytochrome c function. PLoS ONE 2017, 12, e0178280. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Salomon, A.R.; Yu, K.; Doan, J.W.; Grossman, L.I.; Hüttemann, M. New Prospects for an Old Enzyme: Mammalian Cytochrome c Is Tyrosine-Phosphorylated in Vivo. Biochemistry 2006, 45, 9121–9128. [Google Scholar] [CrossRef] [Green Version]
- Hüttemann, M.; Mahapatra, G.; Lee, I.; Grossman, L.I.; Vaishnav, A.; Moraes, C.T.; Ji, Q.Q.; Salomon, A.R.; Edwards, B.F.P. Regulation of cytochrome c by phosphorylation: Mtochondrial respiration and apoptosis. Biophys. J. 2017, 112, 428a. [Google Scholar] [CrossRef]
- Wan, J.; Kalpage, H.A.; Vaishnav, A.; Liu, J.; Lee, I.; Mahapatra, G.; Turner, A.A.; Zurek, M.P.; Ji, Q.; Moraes, C.T.; et al. Regulation of Respiration and Apoptosis by Cytochrome c Threonine 58 Phosphorylation. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Barrios-Maya, M.-A.; Ruiz-Ramírez, A.; Quezada, H.; Acuña, C.L.C.; El-Hafidi, M. Palmitoyl-CoA effect on cytochrome c release, a key process of apoptosis, from liver mitochondria of rat with sucrose diet-induced obesity. Food Chem. Toxicol. 2021, 154, 112351. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Yusoff, N.; Abd Ghani, I.; Othman, N.W.; Aizat, W.M.; Hassan, M. Toxicity and sublethal effect of farnesyl acetate on diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Insects 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, S.R.; Kim, G.H. Insecticidal and antifeeding activity of perilla frutescens derived material against the dia-mondback moth, Plutella xylostella L. Entomol. Res. 2019, 49, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.Y.; Liu, D.Y.; Liu, Z.J.; Shi, J.; He, W.J.; Qi, P.Y.; Chen, J.X.; Song, B.A. Design, synthesis, and insecticidal activity evaluation of novel 4-(N,N-diarylmethylamines)furan-2(5H)-one derivatives as potential acetylcholine receptor insecticide. Pest Manag. Sci. 2019, 75, 427–437. [Google Scholar] [CrossRef] [PubMed]









| Comp. | Plutella xylostella | Toxic Regression Equation | r2 | 95% Confidence Interval (mg/L) | ||
|---|---|---|---|---|---|---|
| 100 (mg/L) (%) | 10 (mg/L) (%) | LC50 (mg/L) | ||||
| 1 | 100 | 100 | 2.53 | y = 3.5287 + 3.6553x | 0.95 | 2.08–3.07 |
| 2 | 100 | 93.3 ± 1.9 | 3.12 | y = 3.5907 + 2.8481x | 0.98 | 2.52–3.88 |
| 3 | 100 | 100 | 1.62 | y = 4.4044 + 2.8452x | 0.95 | 1.26–2.07 |
| 4 | 100 | 86.7 ± 1.9 | 5.21 | y = 2.5900 + 3.3612x | 0.98 | 4.29–6.33 |
| 5 | 100 | 0 | 22.33 | y = 0.0224 + 3.6903x | 0.99 | 18.41–27.07 |
| 6 | 100 | 63.3 ± 5.1 | 5.43 | y = −2.6610 + 3.1839x | 0.99 | 4.46–6.61 |
| 7 | 100 | 100 | 0.51 | y = 6.0002 + 3.4342x | 0.97 | 0.42–0.62 |
| 8 | 100 | 60.0 ± 3.3 | 10.94 | y = 1.5668 + 3.3045x | 0.99 | 8.99–13.31 |
| 9 | 100 | 100 | 2.53 | y = 3.5287 + 3.6553x | 0.95 | 2.08–3.07 |
| 10 | 100 | 0 | 47.51 | y = 0.7868 + 2.5127x | 0.94 | 35.05–64.40 |
| 11 | 100 | 100 | 3.50 | y = 3.3107 + 3.1018x | 0.96 | 2.80–4.39 |
| 12 | 100 | 50.0 ± 3.3 | 4.79 | y = 2.5889 + 3.5447x | 0.96 | 3.94–5.82 |
| 13 | 100 | 43.3 ± 1.9 | 29.15 | y = −2.1821 + 4.9038x | 0.94 | 24.63–34.49 |
| 14 | 100 | 80.0 ± 3.3 | 9.28 | y = 1.6960 + 3.4151x | 0.97 | 7.63–11.28 |
| 15 | 100 | 93.3 ± 1.9 | 7.31 | y = 2.2710 + 3.1592x | 0.95 | 5.79–9.22 |
| 16 | 100 | 60.0 ± 3.3 | 4.07 | y = 3.0852 + 3.1422x | 0.96 | 3.29–5.03 |
| 17 | 100 | 33.3 ± 1.9 | 11.69 | y = 1.2932 + 3.4718x | 0.96 | 9.53–14.33 |
| 18 | 100 | 63.3 ± 3.9 | 13.63 | y = 1.4488 + 3.1302x | 0.96 | 10.98–16.93 |
| 19 | 100 | 3.3 ± 1.9 | 34.23 | y = −1.4041 + 4.1737x | 0.97 | 29.40–39.86 |
| 20 | 100 | 0 | 42.00 | y = −1.0729 + 3.7413x | 0.95 | 35.27–50.01 |
| 21 | 100 | 100 | 2.55 | y = 3.7949 + 2.9582x | 0.99 | 2.10–3.11 |
| 22 | 100 | 100 | 0.89 | y = 5.1782 + 3.4151x | 0.97 | 0.73–1.08 |
| 23 | 100 | 100 | 2.11 | y = 3.9477 + 3.2530x | 0.97 | 1.73–2.57 |
| 24 | 100 | 90.0 ± 3.3 | 5.70 | y = 2.5702 + 3.2151x | 0.99 | 4.67–6.95 |
| 25 | 100 | 83.3 ± 3.9 | 6.11 | y = 2.6955 + 2.9329x | 0.92 | 4.70–7.94 |
| 26 | 100 | 53.3 ± 1.9 | 9.90 | y = 1.3615 + 3.6553x | 0.95 | 8.13–12.04 |
| 27 | 100 | 0 | 37.06 | y = −1.0217 + 3.8381x | 0.96 | 31.55–43.54 |
| 28 | 100 | 90.0 ± 3.3 | 5.34 | y = 2.4222 + 3.5447x | 0.96 | 4.39–6.49 |
| 29 | 100 | 100 | 1.02 | y = 4.9732 + 3.3045x | 0.97 | 0.84–1.24 |
| 30 | 100 | 53.3 ± 1.9 | 15.77 | y = 1.4866 + 2.9329x | 0.93 | 12.25–20.31 |
| 31 | 100 | 0 | 59.89 | y = −11.5058 + 9.2866x | 0.99 | 55.83–64.25 |
| 32 | 100 | 100 | 0.26 | y = 6.7176 + 2.9582x | 0.99 | 0.22–0.32 |
| Ethiprole | 100 | 53.3 ± 1.9 | 3.81 | y = −1.0781 + 1.8553x | 0.97 | 2.47–6.06 |
| Avermectin | 100 | 30.0 ± 3.3 | 12.32 | y = −4.7237+4.3311x | 0.96 | 9.70–16.24 |
| Statistical Parameter | CoMFA | CoMSIA | Verification Standard |
|---|---|---|---|
| q2a | 0.751 | 0.697 | >0.5 |
| ONCb | 4 | 10 | |
| r2c | 0.931 | 0.977 | >0.8 |
| SEEd | 0.163 | 0.106 | |
| Fe | 90.860 | 90.132 | |
| Fraction of Field Contributions | |||
| steric | 0.527 | 0.095 | |
| electrostatic | 0.473 | 0.291 | |
| hydrophobic | 0.226 | ||
| hydrogen-bond acceptor | 0.291 | ||
| hydrogen-bond donor | 0.097 | ||
| Comp. | Experimental (pLC50) | CoMFA | CoMSIA | ||
|---|---|---|---|---|---|
| Predict a | Residual b | Predict a | Residual b | ||
| 1 | 5.338 | 5.397 | 0.059 | 5.344 | 0.006 |
| 2 | 5.260 | 5.233 | −0.027 | 5.258 | −0.002 |
| 3 | 5.545 | 5.957 | 0.412 | 5.674 | 0.129 |
| 4 | 5.038 | 4.923 | −0.115 | 5.043 | 0.005 |
| 5 | 4.418 | 4.723 | 0.305 | 4.479 | 0.061 |
| 6 | 5.032 | 4.977 | −0.055 | 5.081 | 0.049 |
| 7 | 6.059 | 5.932 | −0.127 | 6.005 | −0.054 |
| 8 | 4.759 | 4.899 | 0.140 | 4.887 | 0.128 |
| 9 | 5.395 | 5.388 | −0.007 | 5.455 | 0.060 |
| 10 c | 4.114 | 4.101 | −0.013 | 4.126 | 0.012 |
| 11 | 5.247 | 5.009 | −0.238 | 5.176 | −0.071 |
| 12 c | 5.111 | 4.924 | −0.187 | 5.205 | 0.094 |
| 13 | 4.287 | 4.431 | 0.144 | 4.413 | 0.126 |
| 14 | 4.784 | 4.554 | −0.230 | 4.678 | −0.106 |
| 15 c | 4.898 | 5.007 | 0.109 | 4.866 | −0.032 |
| 16 | 5.153 | 4.927 | −0.226 | 5.131 | −0.022 |
| 17 | 4.696 | 4.703 | 0.007 | 4.646 | −0.050 |
| 18 | 4.668 | 4.812 | 0.144 | 4.827 | 0.159 |
| 19 | 4.245 | 4.247 | 0.002 | 4.217 | −0.028 |
| 20 | 4.187 | 4.187 | −0.000 | 4.166 | −0.021 |
| 21 | 5.362 | 5.379 | 0.017 | 5.366 | 0.004 |
| 22 | 5.819 | 5.943 | 0.124 | 5.792 | −0.027 |
| 23 | 5.444 | 5.472 | 0.028 | 5.391 | −0.053 |
| 24 | 5.025 | 4.923 | −0.102 | 5.018 | −0.007 |
| 25 | 4.995 | 4.86 | −0.135 | 4.687 | −0.308 |
| 26 c | 4.772 | 4.826 | 0.054 | 4.687 | −0.085 |
| 27 | 4.198 | 4.224 | 0.026 | 4.242 | 0.044 |
| 28 c | 5.025 | 4.918 | −0.107 | 5.083 | 0.058 |
| 29 c | 5.779 | 5.812 | 0.033 | 5.734 | −0.045 |
| 30 | 4.590 | 4.693 | 0.103 | 4.535 | −0.055 |
| 31 | 4.021 | 4.132 | 0.111 | 4.06 | 0.039 |
| 32 d | 6.360 | 6.113 | −0.247 | 6.348 | −0.012 |
| Protein ID | Protein Names | Gene Names | Sig |
|---|---|---|---|
| A0A023HN92_PLUXY | Mitochondrial cytochrome C | PLXY2_LOCUS4707 | down |
| Q60FR7_PLUXY | Vacuolar ATP synthethase subunit e | no | |
| D5LN47_PLUXY | ATP synthase subunit d | PLXY2_LOCUS145 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Jiang, B.; Luo, Y.; He, S.; Feng, D.; Hu, D.; Song, R. Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella. Molecules 2023, 28, 3300. https://doi.org/10.3390/molecules28083300
Li F, Jiang B, Luo Y, He S, Feng D, Hu D, Song R. Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella. Molecules. 2023; 28(8):3300. https://doi.org/10.3390/molecules28083300
Chicago/Turabian StyleLi, Fangyi, Biaobiao Jiang, Yuqin Luo, Siqi He, Di Feng, Deyu Hu, and Runjiang Song. 2023. "Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella" Molecules 28, no. 8: 3300. https://doi.org/10.3390/molecules28083300