Maltooligosaccharides: Properties, Production and Applications
Abstract
:1. Introduction
2. Properties of Maltooligosaccharides
3. Application of Maltooligosaccharides
3.1. Maltooligosaccharides in Their Natural Structure
3.2. Derivatives of Maltooligosaccharides
3.2.1. Maltooligosaccharide Derivatives for Spectrophotometric Determination of Enzyme Activity
3.2.2. Alkylmaltooligosaccharides
3.2.3. Sulfated Alkylmaltooligosaccharides
3.2.4. Sulfated Maltooligosaccharides
3.2.5. Other Derivatives of Maltooligosaccharides
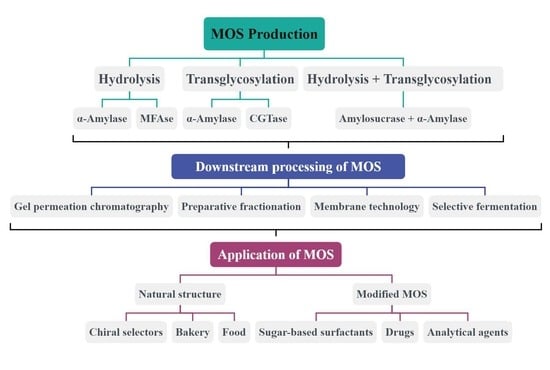
4. Overview of Different Ways of Producing Maltooligosaccharides
4.1. Preparation of MOS by Hydrolytic Reactions
4.1.1. α-Amylases
4.1.2. Maltooligosaccharide-Forming Amylases
4.2. Preparation of MOS by Transglycosylation Reaction
4.2.1. α-Amylases
4.2.2. Cyclodextrin Glycosyltransferases
4.3. Preparation of MOS Combining Hydrolytic and Transglycosylation Reactions
5. Preparation of Maltooligosaccharide Derivatives
5.1. Maltooligosaccharide Chromogenic or Fluorogenic Substrates
5.2. Alkylmaltooligosaccharides
6. Downstream Processing of Maltooligosaccharides
7. Immobilization of Enzymes for the Production of Maltooligosaccharides
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sim, L.; Quezada-Calvillo, R.; Sterchi, E.E.; Nichols, B.L.; Rose, D.R. Human Intestinal Maltase-Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity. J. Mol. Biol. 2008, 375, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.Y.; Hong, K.B.; Chang, Y.B.; Shin, J.; Jung, E.Y.; Jo, K.; Suh, H.J. In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules 2020, 25, 5201. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.O. Functional Oligosaccharide: Chemicals Structure, Manufacturing, Health Benefits, Applications and Regulations. J. Food Chem. Nanotechnol. 2018, 4, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wu, J.; Zhang, B.; Wang, F.; Ye, X.; Huang, Y.; Huang, Q.; Cui, Z. AmyM, a novel maltohexaose-forming α-amylase from Corallococcus sp. strain EGB. Appl. Environ. Microbiol. 2015, 81, 1977–1987. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Wei, X.; Sun, C.; Chi, M.; Bi, W.; Yang, B.; et al. Effect of Functional Oligosaccharides and Ordinary Dietary Fiber on Intestinal Microbiota Diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Ding, N.; Ren, J.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Holler, T.P.; Li, Z. Maltooligosaccharide-forming amylase: Characteristics, preparation, and application. Biotechnol. Adv. 2017, 35, 619–632. [Google Scholar] [CrossRef]
- Lekakarn, H.; Bunterngsook, B.; Pajongpakdeekul, N.; Prongjit, D.; Champreda, V. A novel low temperature active maltooligosaccharides-forming amylase from Bacillus koreensis HL12 as biocatalyst for maltooligosaccharide production. 3 Biotech 2022, 12, 134. [Google Scholar] [CrossRef]
- Nakakuki, T. Present status and future of functional oligosaccharide development in Japan. Pure Appl. Chem. 2002, 74, 1245–1251. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Li, Q. Simultaneous Determination of Maltooligosaccharides in Beer Using HPLC-ELSD and Their Influence on Beer Foam Stability. J. Am. Soc. Brew. Chem. 2015, 73, 78–83. [Google Scholar] [CrossRef]
- Li, J.; Ban, X.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Li, Z. Preparation and antibacterial activity of a novel maltotetraose product. Process Biochem. 2021, 108, 8–17. [Google Scholar] [CrossRef]
- Shinde, V.K.; Vamkudoth, K.R. Maltooligosaccharide forming amylases and their applications in food and pharma industry. J. Food Sci. Technol. 2021, 59, 3733–3744. [Google Scholar] [CrossRef] [PubMed]
- Pélingre, M.; Koffi Teki, D.S.-E.; El-Abid, J.; Chagnault, V.; Kovensky, J.; Bonnet, V. Ring-Opening of Cyclodextrins: An Efficient Route to Pure Maltohexa-, Hepta-, and Octaoses. Organics 2021, 2, 287–305. [Google Scholar] [CrossRef]
- Lim, J.; Pullicin, A.J. Oral carbohydrate sensing: Beyond sweet taste. Physiol. Behav. 2019, 202, 14–25. [Google Scholar] [CrossRef]
- Kearsley, M.; Dziedzic, S. Handbook of Starch Hydrolysis Products and Their Derivatives; Springer Science & Business Media: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Barresi, F.; Eads, A.; Keuyon, M. Maltooligosaccharides from Corn. Oligosacch. Food Agric. 2003, 14, 182–195. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.; Tzur, D.; Knox, C.; Eisner, R.; AC, G.A.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef]
- Hashim, S.O. Starch-Modifying Enzymes. Adv. Biochem. Eng. Biotechnol. 2020, 172, 221–244. [Google Scholar] [CrossRef]
- Aiyer, P.V. Amylases and their applications. Afr. J. Biotechnol. 2005, 4, 1525–1529. [Google Scholar] [CrossRef]
- Hebeda, R.E.; Bowles, L.K.; Teague, W.M. Use of intermediate temperature stability enzymes for retarding staling in baked goods. Cereal Foods World 1991, 36, 619–624. [Google Scholar]
- Smits, A.L.M.; Kruiskamp, P.H.; Van Soest, J.J.G.; Vliegenthart, J.F.G. The influence of various small plasticisers and malto-oligosaccharides on the retrogradation of (partly) gelatinised starch. Carbohydr. Polym. 2003, 51, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Soini, H.; Stefansson, M.; Riekkola, M.-L.; Novotny, M. V Maltooligosaccharides as Chiral Selectors for the Separation of Pharmaceuticals by Capillary Electrophoresis. Anal. Chem. 2002, 66, 3477–3484. [Google Scholar] [CrossRef]
- Taichi Usui, S.; Teruo Nakakuki, M.; Kazuo Sakai, Y. Process for the Preparation of Derivatives of Maltooligosaccharides. U.S. Patent Application No. US5208151, 4 May 1993. [Google Scholar]
- Li, S.L.; Wang, Y.H.; Chao, Y.C.; Bai, M.D.; Cheng, S.S. Amylolysis is predominated by cell-surface-bound hydrolase during anaerobic fermentation under mesophilic conditions. J. Biosci. Bioeng. 2018, 125, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Arai, E. Improvement of gluten-free steamed bread quality by partial substitution of rice flour with powder of Apios americana tuber. Food Chem. 2021, 337, 127977. [Google Scholar] [CrossRef]
- Mezgebe, A.G.; Abegaz, K.; Taylor, J.R.N. Relationship between waxy (high amylopectin) and high protein digestibility traits in sorghum and malting quality. J. Cereal Sci. 2018, 79, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.; Liu, J.; Hu, S.; Dong, J.; Yu, J.; Fang, L.; Huang, S.; Wang, L. Comparative proteomic analysis of different barley cultivars during seed germination. J. Cereal Sci. 2021, 102, 103357. [Google Scholar] [CrossRef]
- Bai, Y.; Atluri, S.; Zhang, Z.; Gidley, M.J.; Li, E.; Gilbert, R.G. Structural reasons for inhibitory effects of pectin on α-amylase enzyme activity and in-vitro digestibility of starch. Food Hydrocoll. 2021, 114, 106581. [Google Scholar] [CrossRef]
- Tagomori, B.Y.; dos Santos, F.C.; Barbosa-Tessmann, I.P. Recombinant expression, purification, and characterization of an α-amylase from Massilia timonae. 3 Biotech 2021, 11, 13. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wang, G.; Cao, J.; Yang, X.; Liu, X.; Sun, L. α-Amylase inhibition of a certain dietary polyphenol is predominantly affected by the concentration of α-1, 4-glucosidic bonds in starchy and artificial substrates. Food Res. Int. 2022, 157, 111210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.; Moore, A.R.; Conway, R.; Zeppelin, T.; Gelatt, T.; Duncan, C. Establishing a reference interval for acute phase proteins, cytokines, antioxidants and commonly measured biochemical and hematologic parameters in the northern fur seal (Callorhinus ursinus). Vet. Immunol. Immunopathol. 2021, 242, 110348. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Makino, Y.; Omichi, K. Sensitive assay of glycogen phosphorylase activity by analysing the chain-lengthening action on a fluologenic maltooligosaccharide derivative. J. Biochem. 2009, 146, 71–76. [Google Scholar] [CrossRef]
- Cheng, A.; Zhang, M.; Okubo, M.; Omichi, K.; Saltiel, A.R. Distinct mutations in the glycogen debranching enzyme found in glycogen storage disease type III lead to impairment in diverse cellular functions. Hum. Mol. Genet. 2009, 18, 2045–2052. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, E.; Makino, Y.; Omichi, K. Active site mapping of amylo-α-1,6-glucosidase in porcine liver glycogen debranching enzyme using fluorogenic 6-O-α-glucosyl- maltooligosaccharides. J. Biochem. 2007, 141, 627–634. [Google Scholar] [CrossRef]
- Cornaggia, C.; Ivory, R.; Mangan, D.; Mccleary, B.V. Novel assay procedures for the measurement of α-amylase in weather-damaged wheat. J. Sci. Food Agric. 2015, 96, 404–412. [Google Scholar] [CrossRef]
- McCleary, B.V.; Mangan, D.; McKie, V.; Cornaggia, C.; Ivory, R.; Rooney, E. Colourimetric and fluorometric substrates for measurement of pullulanase activity. Carbohydr. Res. 2014, 393, 60–69. [Google Scholar] [CrossRef]
- Mangan, D.; McCleary, B.V.; Cornaggia, C.; Ivory, R.; Rooney, E.; McKie, V. Colourimetric and fluorimetric substrates for the assay of limit dextrinase. J. Cereal Sci. 2015, 62, 50–57. [Google Scholar] [CrossRef]
- Hill, K.; Rhode, O. Sugar-based surfactants for consumer products and technical applications. Lipid-Fett 1999, 101, 25–33. [Google Scholar] [CrossRef]
- Fischer, E. Ueber die Glucoside der Alkohole. Arch. Pharm. 1893, 207, 2400–2412. [Google Scholar] [CrossRef] [Green Version]
- Maggio, E.T. Pharmaceutical Composition Including Alkyl glycoside and an Anti-Seizure Agent. US9642913B2, 9 May 2017. [Google Scholar]
- Koeltzow, D.E.; Urefer, A.D. Preparation and properties of pure alkyl glucosides, maltosides and maltotriosides. J. Am. Oil Chem. Soc. 1984, 61, 1651–1655. [Google Scholar] [CrossRef]
- Ara, K.Z.G.; Linares-Pastén, J.A.; Jönsson, J.; Viloria-Cols, M.; Ulvenlund, S.; Adlercreutz, P.; Karlsson, E.N. Engineering CGTase to improve synthesis of alkyl glycosides. Glycobiology 2021, 31, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Clercq, E. De Chemotherapeutic approaches to the treatment of the acquired immune deficiency syndrome (AIDS). J. Med. Chem. 1986, 29, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Yoshida, O.; Tochikura, T.S.; Yoshida, T.; Mimura, T.; Kido, Y.; Motoki, Y.; Kaneko, Y.; Uryu, T.; Yamamoto, N. Sulfation of polysaccharides generates potent and selective inhibitors of human immunodeficiency virus infection and replication in vitro. Jpn. J. Cancer Res. GANN 1987, 78, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, O.; Nakashima, H.; Yoshida, T.; Kaneko, Y.; Yamamoto, I.; Matsuzaki, K.; Uryu, T.; Yamamoto, N. Sulfation of the immunomodulating polysaccharide lentinan: A novel strategy for antivirals to human immunodeficiency virus (HIV). Biochem. Pharmacol. 1988, 37, 2887–2891. [Google Scholar] [CrossRef]
- Hatanaka, K.; Yoshida, T.; Uryu, T.; Yoshida, O.; Nakashima, H.; Yamamoto, N.; Mimura, T.; Kaneko, Y. Synthesis of an Inhibitor of Human Immunodeficiency Virus Infection. Jpn. J. Cancer Res. 1989, 80, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Katsuraya, K.; Ikushima, N.; Takahashi, N.; Shoji, T.; Nakashima, H.; Yamamoto, N.; Yoshida, T.; Uryu, T. Synthesis of sulfated alkyl malto- and laminara-oligosaccharides with potent inhibitory effects on AIDS virus infection. Carbohydr. Res. 1994, 260, 51–61. [Google Scholar] [CrossRef]
- Gordon, M.; Guralnik, M.; Kaneko, Y.; Mimura, T.; Baker, M.; Lang, W. A phase I study of curdlan sulfate--an HIV inhibitor. Tolerance, pharmacokinetics and effects on coagulation and on CD4 lymphocytes. J. Med. 1994, 25, 163–180. [Google Scholar]
- Yoshida, T. Anti-HIV Mechanism of Sulfated Poly and Oligosaccharides. J. Fiber Sci. Technol. 2020, 76, 387–402. [Google Scholar] [CrossRef]
- Gonçalves, A.G.; Noseda, M.D.; Duarte, M.E.R.; Grindley, T.B. Semisynthesis of long-chain alkyl ether derivatives of sulfated oligosaccharides via dibutylstannylene acetal intermediates. J. Org. Chem. 2007, 72, 9896–9904. [Google Scholar] [CrossRef]
- Hulett, M.D.; Freeman, C.; Hamdorf, B.J.; Baker, R.T.; Harris, M.J.; Parish, C.R. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat. Med. 1999, 5, 803–809. [Google Scholar] [CrossRef]
- Nakajima, M.; Irimura, T.; Di Ferrante, D.; Di Ferrante, N.; Nicolson, G.L. Heparan sulfate degradation: Relation to tumor invasive and metastatic properties of mouse B16 melanoma sublines. Science 1983, 220, 611–613. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Elkin, M.; Pappo, M.; Aingorn, H.; Atzmon, R.; Ishai-Michaeli, R.; Aviv, A.; Pecker, I.; Friedmann, Y. Mammalian heparanase as mediator of tumor metastasis and angiogenesis. Isr. Med. Assoc. J. 2000, 2, 37–45. [Google Scholar] [PubMed]
- Campbell, J.H.; Rennick, R.E.; Kalevitch, S.G.; Campbell, G.R. Heparan sulfate-degrading enzymes induce modulation of smooth muscle phenotype. Exp. Cell Res. 1992, 200, 156–167. [Google Scholar] [CrossRef]
- Ferro, V.; Hammond, E.; Fairweather, J. The Development of Inhibitors of Heparanase, a Key Enzyme Involved in Tumour Metastasis, Angiogenesis and Inflammation. Mini-Rev. Med. Chem. 2004, 4, 693–702. [Google Scholar] [CrossRef]
- Tressler, R.J.; Wee, J.; Storm, N.; Fugedi, P.; Peto, C.; Stack, R.J.; Tyrrell, D.J.; Killion, J.J. A Heparanase-Inhibitory, bFGF-Binding Sulfated Oligosaccharide that Inhibits Angiogenesis Ex Ovo has Potent Antitumor and Antimetastatic Activity in Vivo. Mol. Cell. Clin. Asp. Angiogenes. 1996, 285, 199–211. [Google Scholar] [CrossRef]
- Katsuraya, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Synthesis of sulfated oligosaccharide glycosides having high anti-HIV activity and the relationship between activity and chemical structure. Carbohydr. Res. 1999, 315, 234–242. [Google Scholar] [CrossRef]
- Yoshida, T.; Akasaka, T.; Choi, Y.; Hattori, K.; Yu, B.; Mimura, T.; Kaneko, Y.; Nakashima, H.; Aragaki, E.; Premanathan, M.; et al. Synthesis of polymethacrylate derivatives having sulfated maltoheptaose side chains with anti-HIV activities. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 789–800. [Google Scholar] [CrossRef]
- Tagami, T.; Tanaka, Y.; Mori, H.; Okuyama, M.; Kimura, A. Enzymatic synthesis of acarviosyl-maltooligosaccharides using disproportionating enzyme 1. Biosci. Biotechnol. Biochem. 2013, 77, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, E.; Jánossy, L.; Harangi, J.; Kandra, L.; Lipták, A. Synthesis of chromogenic substrates of α-amylases on a cyclodextrin basis. Carbohydr. Res. 1997, 303, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Dollings, P.J. Benzylmaltotriosides as Inhibitors of Smooth Muscle Cell Proliferation. U.S. Patent WO/2000/031095, 2 June 2000. [Google Scholar]
- Nakamura, M.; Makino, Y.; Takagi, C.; Yamagaki, T.; Sato, M. Probing the catalytic site of rabbit muscle glycogen phosphorylase using a series of specifically modified maltohexaose derivatives. Glycoconj. J. 2017, 34, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.I.; Chigita, H.; Yamamoto, K. Chemoenzymatic synthesis of carboxylate-terminated maltooligosaccharides and their use for cross-linking of chitin. Int. J. Biol. Macromol. 2020, 159, 510–516. [Google Scholar] [CrossRef]
- Thieme, R.; Lay, H.; Oser, A.; Lehmann, J.; Wrissenberg, S.; Winfried, B. 3-Azi-l-methoxybutyl D-maltooligosaccharides specifically bind to the maltose/maltooligosaccharide-binding protein of Escherichia coli and can be used as photoaffinity labels. Eur. J. Biochem. 1986, 160, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Umegatani, Y.; Izawa, H.; Nawaji, M.; Yamamoto, K.; Kubo, A.; Yanase, M.; Takaha, T.; Kadokawa, J.I. Enzymatic α-glucuronylation of maltooligosaccharides using α-glucuronic acid 1-phosphate as glycosyl donor catalyzed by a thermostable phosphorylase from Aquifex aeolicus VF5. Carbohydr. Res. 2012, 350, 81–85. [Google Scholar] [CrossRef]
- Kawazoe, S.; Izawa, H.; Nawaji, M.; Kaneko, Y.; Kadokawa, J. ichi Phosphorylase-catalyzed N-formyl-α-glucosaminylation of maltooligosaccharides. Carbohydr. Res. 2010, 345, 631–636. [Google Scholar] [CrossRef]
- Nawaji, M.; Izawa, H.; Kaneko, Y.; Kadokawa, J. ichi Enzymatic α-glucosaminylation of maltooligosaccharides catalyzed by phosphorylase. Carbohydr. Res. 2008, 343, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Degn, P.; Larsen, K.L.; Duus, J.; Petersen, B.O.; Zimmermann, W. Two-step enzymatic synthesis of maltooligosaccharide esters. Carbohydr. Res. 2000, 329, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Ogawa, K.; Saito, S.; Murata, T.; Usui, T. Chemo-enzymatic synthesis of galactosylmaltooligosaccharidonolactone as a substrate analogue inhibitor for mammalian α-amylase. J. Biochem. 1998, 123, 508–515. [Google Scholar] [CrossRef]
- Yasukawa, R.; Moriwaki, N.; Uesugi, D.; Kaneko, F.; Hamada, H.; Ozaki, S.I. Enzymatic Synthesis of Quercetin Monoglucopyranoside and Maltooligosaccharides. Nat. Prod. Commun. 2015, 10, 949–950. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Koh, D.W.; Territo, P.R.; Park, C.S.; Hamaker, B.R.; Yoo, S.H. Enzymatic synthesis of 2-deoxyglucose-containing maltooligosaccharides for tracing the location of glucose absorption from starch digestion. Carbohydr. Polym. 2015, 132, 41–49. [Google Scholar] [CrossRef]
- Satomura, S.; Omichi, K.; Ikenaka, T. Preparation of carboxymethyl derivatives of p-nitrophenyl alpha-maltopentaoside as substrate of alpha-amylase. Carbohydr. Res. 1988, 180, 137–146. [Google Scholar] [CrossRef]
- Sauvageot, N.; Mokhtari, A.; Joyet, P.; Budin-Verneuil, A.; Blancato, V.S.; Repizo, G.D.; Henry, C.; Pikis, A.; Thompson, J.; Magni, C.; et al. Enterococcus faecalis uses a phosphotransferase system permease and a host colonization-related ABC transporter for maltodextrin uptake. J. Bacteriol. 2017, 199, e00878-16. [Google Scholar] [CrossRef] [Green Version]
- Laboureau, J.; Ballihaut, C. Utilisation D’oligosaccharides Phosphoryles Pour Reduire L’apparence et/ou la Visibilite des Pores de la Peau. FR1253880A, 1 November 2013. [Google Scholar]
- Kandra, L.; Gyémánt, G.; Pál, M.; Petró, M.; Remenyik, J.; Lipták, A. Chemoenzymatic synthesis of 2-chloro-4-nitrophenyl β-maltoheptaoside acceptor-products using glycogen phosphorylase b. Carbohydr. Res. 2001, 333, 129–136. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shoda, S.; Kashiwa, K.M.D.O.; Shimada, J. Method for the Preparation of Malto-Oligosaccharide. U.S. Patent EP0530421A1, 10 March 1993. [Google Scholar]
- Marchal, L.M.; Beeftink, H.H.; Tramper, J. Towards a rational design of commercial maltodextrins. Trends Food Sci. Technol. 1999, 10, 345–355. [Google Scholar] [CrossRef]
- Omoto, S.; Shomura, T.; Inoue, S.; Nida, T.; Hisamatsu, T.; Uchida, S. Process for Production of Maltopentaose and Maltohexaose. US4151041A, 24 April 1979. [Google Scholar]
- Kimura, T.; Ogata, M.; Kobayashi, H.; Yoshida, M.; Oishi, K.; Nakakuki, T. Continuous production of maltotetraose using a dual immobilized enzyme system of maltotetraose-forming amylase and pullulanase. Biotechnol. Bioeng. 1990, 36, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, T.J. Process for Producing Maltopentaose. US4039383A, 2 August 1977. [Google Scholar]
- Moon, I.S.; Cho, G. Production of maltooligosaccharides from starch and separation of maltopentaose by adsorption of them on activated carbon (I). Biotechnol. Bioprocess Eng. 1997, 2, 19–22. [Google Scholar] [CrossRef]
- Rahmani, N.; Rohana, R.; Sukarno, S.; Andriani, A.; Yopi, Y. Production of Maltooligosaccharides from Black Potato (Coleus tuberosus) Starch by α-amylase from a Marine Bacterium (Brevibacterium sp.). Microbiol. Indones. 2013, 7, 6. [Google Scholar] [CrossRef]
- Prakash, B.; Vidyasagar, M.; Madhukumar, M.S.; Muralikrishna, G.; Sreeramulu, K. Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem. 2009, 44, 210–215. [Google Scholar] [CrossRef]
- Chakraborty, S.; Khopade, A.; Biao, R.; Jian, W.; Liu, X.Y.; Mahadik, K.; Chopade, B.; Zhang, L.; Kokare, C. Characterization and stability studies on surfactant, detergent and oxidant stable α-amylase from marine haloalkaliphilic Saccharopolyspora sp. A9. J. Mol. Catal. B Enzym. 2011, 68, 52–58. [Google Scholar] [CrossRef]
- Jana, M.; Maity, C.; Samanta, S.; Pati, B.R.; Islam, S.S.; Mohapatra, P.K.D.; Mondal, K.C. Salt-independent thermophilic α-amylase from Bacillus megaterium VUMB109: An efficacy testing for preparation of maltooligosaccharides. Ind. Crops Prod. 2013, 41, 386–391. [Google Scholar] [CrossRef]
- Park, J.T.; Suwanto, A.; Tan, I.; Nuryanto, T.; Lukman, R.; Wang, K.; Jane, J. lin Molecular cloning and characterization of a thermostable α-amylase exhibiting an unusually high activity. Food Sci. Biotechnol. 2014, 23, 125–132. [Google Scholar] [CrossRef]
- Hmidet, N.; Maalej, H.; Haddar, A.; Nasri, M. A novel α-Amylase from Bacillus mojavensis A21: Purification and biochemical characterization. Appl. Biochem. Biotechnol. 2010, 162, 1018–1030. [Google Scholar] [CrossRef]
- Iefuji, H.; Chino, M.; Kato, M.; Iimura, Y. Raw-starch-digesting and thermostable α-amylase from the yeast Cryptococcus sp. S-2: Purification, characterization, cloning and sequencing. Biochem. J. 1996, 318, 989–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino, A.C.M.M.; Jorge, J.A.; Terenzi, H.F.; Polizeli, M.L.T.M. Studies on a thermostable α-amylase from the thermophilic fungus Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 2003, 61, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Doukyu, N.; Yamagishi, W.; Kuwahara, H.; Ogino, H.; Furuki, N. Purification and characterization of a maltooligosaccharide-forming amylase that improves product selectivity in water-miscible organic solvents, from dimethylsulfoxide-tolerant Brachybacterium sp. strain LB25. Extremophiles 2007, 11, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Palit, S.; Banerjee, R.; Maiti, B. Purification and characterization of maltooligosaccharide-forming amylase from Bacillus circulans GRS 313. J. Ind. Microbiol. Biotechnol. 2002, 28, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.R.; Rajagopalan, G.; Krishnan, C. Purification and characterization of a maltooligosaccharide-forming α-amylase from a new Bacillus subtilis KCC103. Appl. Microbiol. Biotechnol. 2006, 73, 591–597. [Google Scholar] [CrossRef]
- Ozturk, H.U.; Denizci, A.A.; Ogan, A.; Kazan, D. A Maltooligosaccharides Producing α-Amylase from Bacillus subtilis SDP1 Isolated from Rhizosphere of Acacia cyanophylla Lindley. Food Biotechnol. 2014, 28, 309–332. [Google Scholar] [CrossRef]
- Messaoud, E.B.; Ali, M.B.; Elleuch, N.; Masmoudi, N.F.; Bejar, S. Purification and properties of a maltoheptaose- and maltohexaose-forming amylase produced by Bacillus subtilis US116. Enzym. Microb. Technol. 2004, 34, 662–666. [Google Scholar] [CrossRef]
- Kashiwagi, N.; Miyake, M.; Hirose, S.; Sota, M.; Ogino, C.; Kondo, A. Cloning and starch degradation profile of maltotriose-producing amylases from Streptomyces species. Biotechnol. Lett. 2014, 36, 2311–2317. [Google Scholar] [CrossRef]
- Yang, C.H.; Liu, W.H. Cloning and characterization of a maltotriose-producing α-amylase gene from Thermobifida fusca. J. Ind. Microbiol. Biotechnol. 2007, 34, 325–330. [Google Scholar] [CrossRef]
- Champreda, V.; Kanokratana, P.; Sriprang, R.; Tanapongpipat, S.; Eurwilaichitr, L. Purification, biochemical characterization, and gene cloning of a new extracellular thermotolerant and glucose tolerant maltooligosaccharide-forming α-amylase from an endophytic ascomycete Fusicoccum sp. BCC4124. Biosci. Biotechnol. Biochem. 2007, 71, 2010–2020. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, D.J.; Choi, Y.L. Characterization of maltotriose production by hydrolyzing of soluble starch with α-amylase from Microbulbifer thermotolerans DAU221. Appl. Microbiol. Biotechnol. 2015, 99, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Kamon, M.; Sumitani, J.I.; Tani, S.; Kawaguchi, T.; Kamon, M.; Sumitani, J.; Tani, S.; Kawaguchi, T. Characterization and gene cloning of a maltotriose-forming exo-amylase from Kitasatospora sp. MK-1785. Appl. Microbiol. Biotechnol. 2015, 99, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, Y. An amylase producing maltotriose from bacillus sub tills. Agric. Biol. Chem. 1985, 49, 1091–1097. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kanai, H.; Hayashi, T.; Akiba, T.; Akaboshi, R.; Horikoshi, K. Haloalkaliphilic maltotriose-forming α-amylase from the archaebacterium Natronococcus sp. strain Ah-36. J. Bacteriol. 1992, 174, 3439–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S.; Nagasaki, K.; Nishimoto, H.; Shigematu, R.; Umesaki, J.; Takenaka, S.; Kaulpiboon, J.; Prousoontorn, M.; Limpaseni, T.; Pongsawasdi, P.; et al. Purification and characterization of five alkaline, thermotolerant, and maltotetraose-producing α-amylases from Bacillus halodurans MS-2-5, and production of recombinant enzymes in Escherichia coli. Enzym. Microb. Technol. 2008, 43, 321–328. [Google Scholar] [CrossRef]
- Maalej, H.; Ben Ayed, H.; Ghorbel-Bellaaj, O.; Nasri, M.; Hmidet, N. Production and biochemical characterization of a high maltotetraose (g4) producing amylase from Pseudomonas stutzeri AS22. Biomed Res. Int. 2014, 2014, 156438. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Khare, S.K. Purification and characterization of maltooligosaccharide-forming α-amylase from moderately halophilic Marinobacter sp. EMB8. Bioresour. Technol. 2012, 116, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Byun, S.M. A novel maltotetraose-forming alkaline α-amylase from an alkalophilic Bacillus strain, GM8901. Prog. Biotechnol. 1996, 12, 61–82. [Google Scholar]
- Ratanakhanokchai, K.; Kaneko, J.; Kamio, Y.; Izaki, K. Purification and properties of a maltotetraose- and maltotriose-producing amylase from Chloroflexus aurantiacus. Appl. Environ. Microbiol. 1992, 58, 2490–2494. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Baba, T.; Takano, T.; Kobayashi, S.; Arai, Y. Nucleotide sequence of the maltotetraohydrolase gene from Pseudomonas saccharophila. FEBS Lett. 1989, 255, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, W.M.; Bourke, A.C.; Kelly, C.T.; Doyle, E.M. A constitutive maltotetraose-producing amylase from Pseudomonas sp. IMD 353. Appl. Microbiol. Biotechnol. 1994, 42, 198–203. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takaki, Y.; Kobata, K.; Takami, H.; Inoue, A. Characterization of α-maltotetraohydrolase produced by Pseudomonas sp. MS300 isolated from the deepest site of the Mariana Trench. Extremophiles 1998, 2, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Torigoe, K.; Nakada, T.; Tsusaki, K.; Kubota, M.; Sakai, S.; Tsujisaka, Y. Cloning and Nucleotide Sequence of the Gene (amyP) for Maltotetraose-Forming Amylase from Pseudomonas stutzeri MO-19. J. Bacteriol. 1989, 171, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Jin, T.; Xie, X.; Ban, X.; Li, C.; Hong, Y.; Cheng, L.; Gu, Z.; Li, Z. Structure of maltotetraose-forming amylase from Pseudomonas saccharophila STB07 provides insights into its product specificity. Int. J. Biol. Macromol. 2020, 154, 1303–1313. [Google Scholar] [CrossRef]
- Hatada, Y.; Masuda, N.; Akita, M.; Miyazaki, M.; Ohta, Y.; Horikoshi, K. Oxidatively stable maltopentaose-producing α-amylase from a deep-sea Bacillus isolate, and mechanism of its oxidative stability validated by site-directed mutagenesis. Enzym. Microb. Technol. 2006, 39, 1333–1340. [Google Scholar] [CrossRef]
- Shida, O.; Takano, T.; Takagi, H.; Kobayashi, S.; Kadowaki, K. Cloning and Nucleotide Sequence of the Maltopentaose-forming Amylase Gene from Pseudomonas sp. KO-8940. Biosci. Biotechnol. Biochem. 1992, 56, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Morgan, F.J.; Priest, F.G. Characterization of a Thermostable a-Amylase f rom Bacillus licheniformis N CI B 6346. J. Appl. Bacteriol. 1981, 50, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, S.; Jiang, Z.; Liu, S.; Feng, Y.; Gu, Z.; Li, C.; Li, Z. A novel maltooligosaccharide-forming amylase from Bacillus stearothermophilus. Food Biosci. 2019, 30, 100415. [Google Scholar] [CrossRef]
- Kainuma, K.; Kobayashi, S.; Ito, T.; Suzuki, S. Isolation and action pattern of maltohexaose producing amylase from Aerobacter aerogenes. FEBS Lett. 1972, 26, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Hashim, S.O.; Delgado, O.D.; Martínez, M.A.; Kaul, R.H.; Mulaa, F.J.; Mattiasson, B. Alkaline active maltohexaose-forming α-amylase from Bacillus halodurans LBK 34. Enzym. Microb. Technol. 2005, 36, 139–146. [Google Scholar] [CrossRef]
- Kanai, R.; Haga, K.; Akiba, T.; Yamane, K.; Harata, K. Biochemical and crystallographic analyses of maltohexaose-producing amylase from alkalophilic Bacillus sp. 707. Biochemistry 2004, 43, 14047–14056. [Google Scholar] [CrossRef]
- Hayashi, T.; Akiba, T.; Horkoshi, K. Production and Purification of New Maltohexaose-forming Amylases from Alkalophilic Bacillus sp. H-167. Agric. Biol. Chem. 1988, 52, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Momma, M. Cloning and Sequencing of the Maltohexaose-producing Amylase Gene of Klebsiella pneumoniae. Biosci. Biotechnol. Biochem. 2000, 64, 428–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Ali, M.; Mhiri, S.; Mezghani, M.; Bejar, S. Purification and sequence analysis of the atypical maltohexaose-forming α-amylase of the B. stearothermophilus US100. Enzym. Microb. Technol. 2001, 28, 537–542. [Google Scholar] [CrossRef]
- Rodríguez Gastón, J.A.; Costa, H.; Rossi, A.L.; Krymkiewicz, N.; Ferrarotti, S.A. Maltooligosaccharides production catalysed by cyclodextrin glycosyltransferase from Bacillus circulans DF 9R in batch and continuous operation. Process Biochem. 2012, 47, 2562–2565. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, Y.; Xu, W.; Guang, C.; Zhang, W.; Zhang, T.; Mu, W. Bioconversion of sucrose to maltooligosaccharides by the synergistic action of amylosucrase and α-amylase. Process Biochem. 2018, 74, 71–76. [Google Scholar] [CrossRef]
- Crittenden, R.G.; Playne, M.J. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci. Technol. 1996, 7, 353–361. [Google Scholar] [CrossRef]
- Sundarram, A.; Murthy, T.P.K. α -Amylase Production and Applications: A Review. J. Appl. Environ. Microbiol. 2014, 2, 166–175. [Google Scholar] [CrossRef]
- Xie, X.; Ban, X.; Gu, Z.; Li, C.; Hong, Y.; Cheng, L.; Li, Z. Structure-Based Engineering of a Maltooligosaccharide-Forming Amylase To Enhance Product Specificity. J. Agric. Food Chem. 2020, 68, 838–844. [Google Scholar] [CrossRef]
- Hansson, T.; Kaper, T.; Van Oost, J.D.; De Vos, W.M.; Adlercreutz, P. Improved oligosaccharide synthesis by protein engineering of β-glucosidase CelB from hyperthermophilic Pyrococcus furiosus. Biotechnol. Bioeng. 2001, 73, 203–210. [Google Scholar] [CrossRef]
- Hehre, E.J. Enzymatic synthesis of polysaccharide: A biologi-cal type of polymerization. Advan. Enzym. 1951, 11, 297–337. [Google Scholar] [CrossRef]
- Hestrin, S.; Feingold, D.S.; Avigad, G. The mechanism of polysaccharide production from sucrose. 3. Donor–acceptor specificity of levansucrase from Aerobacter levanicum. Biochem. J. 1956, 64, 340–351. [Google Scholar] [CrossRef]
- Bacon, J.; Edelman, J. The action of invertase preparations. Arch. Biochem. 1950, 28, 467–468. [Google Scholar]
- Blanchard, P.; Albon, N. The inversion of sucrose; a complication. Arch. Biochem. 1950, 29, 220–222. [Google Scholar] [PubMed]
- Takano, K.; Miwa, T. Enzymatic transfer of glucose: II. IDENTITY of GLUCOTRANSFERASE and β-glucosidase. J. Biochem. 1950, 37, 435–444. [Google Scholar] [CrossRef]
- Hehre, E.J.; Okada, G.; Genghof, D.S. Glycosylation as the Paradigm of Carbohydrase Action. Adv. Chem. 1973, 117, 309–333. [Google Scholar] [CrossRef]
- Abdul Manas, N.H.; Jonet, M.A.; Abdul Murad, A.M.; Mahadi, N.M.; Illias, R.M. Modulation of transglycosylation and improved malto-oligosaccharide synthesis by protein engineering of maltogenic amylase from Bacillus lehensis G1. Process Biochem. 2015, 50, 1572–1580. [Google Scholar] [CrossRef]
- Teresa, M.M.; Alcalde, M.; Plou, F.J.; Dijkhuizen, L.; Ballesteros, A. Synthesis of malto-oligosaccharides via the acceptor reaction catalyzed by cyclodextrin glycosyltransferases. Biocatal. Biotransformation 2001, 19, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Wallenfels, K.; Földi, P.; Niermann, H.; Bender, H.; Linder, D. The enzymic synthesis, by transglucosylation of a homologous series of glycosidically substituted malto-oligosaccharides, and their use as amylase substrates. Carbohydr. Res. 1978, 61, 359–368. [Google Scholar] [CrossRef]
- Usui, T.; Murata, T.; Yabuuchi, Y.; Ogawa, K. Transglycosylation reaction of maltotriose-forming amylase from Streptomyces griseus. Carbohydr. Res. 1993, 250, 57–66. [Google Scholar] [CrossRef]
- Teshima, S.; Hayashi, Y.; Ishimari, K.; Shimada, A. Maltooligosaccharide Derivatives and Reagents for Determination of Amylase Activity. EP0319993A3, 3 July 1991. [Google Scholar]
- Gella, F.J.; Gubern, G.; Vidal, R.; Canalias, F. Determination of total and pancreatic α-amylase in human serum with 2-chloro-4-nitrophenyl-α-D-maltotriosid as substrate. Clin. Chim. Acta 1997, 259, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Kandra, L.; Gyémánt, G.; Farkas, E.; Lipták, A. Action pattern of porcine pancreatic alpha-amylase on three different series of β-maltooligosaccharide glycosides. Carbohydr. Res. 1997, 298, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Koyama, T.; Hatano, K.; Terunuma, D.; Matsuoka, K. Simple and conveniently accessible bi-fluorescence-labeled substrates for amylases. Bioorganic Med. Chem. Lett. 2010, 20, 1969–1971. [Google Scholar] [CrossRef]
- Oka, H.; Koyama, T.; Hatano, K.; Matsuoka, K. Synthetic studies of bi-fluorescence-labeled maltooligosaccharides as substrates for α-amylase on the basis of fluorescence resonance energy transfer (FRET). Bioorganic Med. Chem. 2012, 20, 435–445. [Google Scholar] [CrossRef]
- Rogers, R.G.; Barresi, F.W. Malto-Oligosaccharide Derived Glycosides. US6528629B2, 4 March 2003. [Google Scholar]
- Roth, C.D.; Moser, K.B.; Bomball, W.A. Continuous Process for Making Alkyl Aldosides from Starch or Other Carbohydrates. US4223129, 16 September 1980. [Google Scholar]
- John, M.; Trénel, G.; Dellweg, H. Quantitative chromatography of homologous glucose oligomers and other saccharides using polyacrylamide gel. J. Chromatogr. A 1969, 42, 476–484. [Google Scholar] [CrossRef]
- Dellweg, H.; John, M.; Trenel, G. Gel permeation chromatography of maltooligosaccharides at different temperatures. J. Chromatogr. A 1971, 57, 89–97. [Google Scholar] [CrossRef]
- Kainuma, K.; Nogami, A.; Mercier, C. Gel permeation chromatography of maltooligosaccharides on polyacrylamide gel. J. Chromatogr. 1976, 121, 361–369. [Google Scholar] [CrossRef]
- Adachi, S.; Watanabe, T.; Hashimoto, K. Distribution and dispersion properties in gel chromatographic separation of maltooligosaccharides with hydrophilic vinyl polymer gel. J. Jpn. Soc. Starch Sci. 1989, 36, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Kondo, H.; Nakatani, H.; Hirom, K. Rapid preparation of maltooligosaccharides from cyclodextrins by column chromatography of hydrophilic vinyl polymer gel. Agric. Biol. Chem. 1981, 45, 2369–2370. [Google Scholar] [CrossRef]
- Whistler, L.R.; Durso, F.D. Chromatographic Separation of Sugars on Charcoal. J. Am. Chem. Soc. 1950, 72, 677–679. [Google Scholar] [CrossRef]
- Thoma, J.A.; Wright, H.B.; French, D. Partition chromatography of homologous saccharides on cellulose columns. Arch. Biochem. Biophys. 1959, 85, 452–460. [Google Scholar] [CrossRef]
- Balto, A.S.; Lapis, T.J.; Silver, R.K.; Ferreira, A.J.; Beaudry, C.M.; Lim, J.; Penner, M.H. On the use of differential solubility in aqueous ethanol solutions to narrow the DP range of food-grade starch hydrolysis products. Food Chem. 2016, 197, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Pullicin, A.J.; Ferreira, A.J.; Beaudry, C.M.; Lim, J.; Penner, M.H. Preparation and characterization of isolated low degree of polymerization food-grade maltooligosaccharides. Food Chem. 2018, 246, 115–120. [Google Scholar] [CrossRef]
- Crittenden, R.; Playne, M. Purification of food-grade oligosaccharides using immobilised cells of Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2002, 58, 297–302. [Google Scholar] [CrossRef]
- Yoon, S.H.; Mukerjea, R.; Robyt, J.F. Specificity of yeast (Saccharomyces cerevisiae) in removing carbohydrates by fermentation. Carbohydr. Res. 2003, 338, 1127–1132. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Illanes, A. Membrane technology for the purification of enzymatically produced oligosaccharides. Seperation Funct. Mol. Food By Membr. Technol. 2019, 4, 113–153. [Google Scholar]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Ito, M.; Hibino, K. Immobilization of cyclodextrin glucanotransferase on capillary membrane. J. Ferment. Bioeng. 1994, 77, 259–263. [Google Scholar] [CrossRef]
- Tardioli, P.W.; Zanin, G.M.; de Moraes, F.F. Characterization of Thermoanaerobacter cyclomaltodextrin glucanotransferase immobilized on glyoxyl-agarose. Enzym. Microb. Technol. 2006, 39, 1270–1278. [Google Scholar] [CrossRef]
- Ogunbadejo, B.; Al-Zuhair, S. MOFs as Potential Matrices in Cyclodextrin Glycosyltransferase Immobilization. Molecules 2021, 26, 680. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Ogata, M.; Yoshida, M.; Nakakuki, T. Continuous production of maltotetraose using immobilized Pseudomonas stutzeri amylase. Biotechnol. Bioeng. 1988, 32, 669–676. [Google Scholar] [CrossRef]
- Siso, M.I.G.; Graber, M.; Condoret, J.-S.; Combes, D. Effect of diffusional resistances on the action pattern of immobilized alpha-amylase. J. Chem. Technol. Biotechnol. 1990, 48, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Kumar, T.S.; Rai, S.K.; Roy, J.K. Statistical optimization of Bacillus alcalophilus α-amylase immobilization on iron-oxide magnetic nanoparticles. Biotechnol. Bioprocess Eng. 2010, 15, 984–992. [Google Scholar] [CrossRef]
- Khan, M.J.; Husain, Q.; Ansari, S.A. Polyaniline-assisted silver nanoparticles: A novel support for the immobilization of α-amylase. Appl. Microbiol. Biotechnol. 2013, 97, 1513–1522. [Google Scholar] [CrossRef]
- Bayramoǧlu, G.; Yilmaz, M.; Arica, M.Y. Immobilization of a thermostable α-amylase onto reactive membranes: Kinetics characterization and application to continuous starch hydrolysis. Food Chem. 2004, 84, 591–599. [Google Scholar] [CrossRef]
- Yang, Y.; Chase, H. a Immobilization of alpha-amylase on poly(vinyl alcohol)-coated perfluoropolymer supports for use in enzyme reactors. Biotechnol. Appl. Biochem. 1998, 28, 145–154. [Google Scholar] [PubMed]
- Jen Tien, C.; Huang Chiang, B. Immobilization of α-amylase on a zirconium dynamic membrane. Process Biochem. 1999, 35, 377–383. [Google Scholar] [CrossRef]
- Pereira, S.E.; Fernandes, K.F.; Ulhoa, C.J. Immobilization of Cryptococcus flavus α-amylase on glass tubes and its application in starch hydrolysis. Starch Staerke 2017, 69, 1600189. [Google Scholar] [CrossRef]
- Veesar, I.A.; Solangi, I.B.; Memon, S. Immobilization of α-amylase onto a calix[4]arene derivative: Evaluation of its enzymatic activity. Bioorg. Chem. 2015, 60, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bindu, V.U.; Shanty, A.A.; Mohanan, P.V. Parameters Affecting the Improvement of Properties and Stabilities of Immobilized α-amylase on Chitosan-metal Oxide Composites. Int. J. Biochem. Biophys. 2018, 6, 44–57. [Google Scholar] [CrossRef]
- Kikani, B.A.; Pandey, S.; Singh, S.P. Immobilization of the α-amylase of Bacillus amyloliquifaciens TSWK1-1 for the improved biocatalytic properties and solvent tolerance. Bioprocess Biosyst. Eng. 2013, 36, 567–577. [Google Scholar] [CrossRef]
- Dey, G.; Singh, B.; Banerjee, R. Immobilization of α-Amylase Produced by Bacillus circulans GRS 313. Braz. Arch. Biol. Technol. 2003, 46, 167–176. [Google Scholar] [CrossRef]
- Reshmi, R.; Sanjay, G.; Sugunan, S. Enhanced activity and stability of α-amylase immobilized on alumina. Catal. Commun. 2006, 7, 460–465. [Google Scholar] [CrossRef]
- Ullah, H.; Pervez, S.; Ahmed, S.; Haleem, K.S.; Qayyum, S.; Niaz, Z.; Nawaz, M.A.; Nawaz, F.; Subhan, F.; Tauseef, I. Preparation, characterization and stability studies of cross-linked α-amylase aggregates (CLAAs) for continuous liquefaction of starch. Int. J. Biol. Macromol. 2021, 173, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Torabizadeh, H.; Tavakoli, M.; Safari, M. Immobilization of thermostable α-amylase from Bacillus licheniformis by cross-linked enzyme aggregates method using calcium and sodium ions as additives. J. Mol. Catal. B Enzym. 2014, 108, 13–20. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Bian, Z.; Xu, J.; Zhang, L.; Qiao, M. Physiochemical characterization of α-amylase as crosslinked enzyme aggregates. Catalysts 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef]
- Nadar, S.S.; Muley, A.B.; Ladole, M.R.; Joshi, P.U. Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int. J. Biol. Macromol. 2016, 84, 69–78. [Google Scholar] [CrossRef]
- Kimura, T.; Yoshida, M.; Oishi, K.; Ogata, M.; Nakakuki, T. Immobilization of Exo-Maltotetraohydrolase and Pullulanase. Agric. Biol. Chem. 1989, 53, 1843–1848. [Google Scholar] [CrossRef]
- Kimura, T.; Nakakuki, T. Maltotetraose, A New Saccharide of Tertiary Property. Starch-Stärke 1990, 42, 151–157. [Google Scholar] [CrossRef]
- Nakakuki, T.; Hayashi, T.; Monma, M.; Kawashima, K.; Kainuma, K. Immobilization of the exo-maltohexaohydrolase by the irradiation method. Biotechnol. Bioeng. 1983, 25, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Beliya, E.; Paul, J.S.; Jadhav, S.K. Nanoarmoured α-amylase: A route leading to exceptional stability, catalysis and reusability for industrial applications. Coord. Chem. Rev. 2022, 464, 214557. [Google Scholar] [CrossRef]
- Prestegard, J.H.; Liu, J.; Widmalm, G. Oligosaccharides and Polysaccharides. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2017; pp. 31–40. [Google Scholar]
- Gopinath, S.C.B.; Anbu, P.; Arshad, M.M.; Lakshmipriya, T.; Voon, C.H.; Hashim, U.; Chinni, S.V. Biotechnological processes in microbial amylase production. Biomed Res. Int. 2017, 2017, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Maltotriose | Maltotetraose | Maltopentaose | Maltohexaose | Maltoheptaose | Maltooctaose | |
|---|---|---|---|---|---|---|
| Number of-glucose unit | 3 | 4 | 5 | 6 | 7 | 8 |
| CAS Registry Number | 1109-28-0 | 34612-38-9 | 34620-76-3 | 34620-77-4 | 34620-78-5 | 6156-84-9 |
| Molecular Weight (g·mol−1) | 504.4 | 666.6 | 828.7 | 990.9 | 1153.0 | 1315.1 |
| Water Solubility (g·L−1) | 554 | 350 | 228 | 252 | 272 | 307 |
| Hydrogen Acceptor Count | 16 | 21 | 26 | 31 | 36 | 41 |
| Hydrogen Donor Count | 11 | 14 | 17 | 20 | 23 | 26 |
| Refractivity (m3·mol⁻1) | 100.75 | 133.16 | 165.58 | 197.99 | 230.40 | 262.82 |
| Polarizability (Å3) | 46.65 | 62.12 | 77.13 | 92.08 | 107.11 | 122.77 |
| MOS Derivative | Position of Functional Group | Application | Ref. |
|---|---|---|---|
| Acarviosyl maltooligosaccharides | Non-reducing end | Inhibitors of glycoside hydrolases | [60] |
| Peracetylated maltooligosaccharides | Both non-reducing and reducing end | Important function as research compound Building block for further synthesis of 4-NP and 2-Cl-4NP-ß-glycosides | [61] |
| Benzyl maltotriosides | Reducing end | Smooth muscle cell proliferation inhibitor | [62] |
| Pyridylaminated maltooligosaccharides | Non-reducing end | Activity assay of glycogen phosphorylase and thus study of glycogen phosphorylase mechanism | [33,63] |
| Carboxylate-terminated maltooligosaccharides | Both non-reducing and reducing end | Cross-linkers of water-soluble chitin to form hydrogel | [64] |
| 3-Azi-1-methoxybutyl-D-maltooligosaccharides | Reducing end | Inhibition of maltose uptake via the maltose-binding protein-dependent transport system in Escherichia coli | [65] |
| α-glucuronylated maltooligosaccharides | Non-reducing end | Represent anionic oligosaccharides useful in glycomaterials | [66] |
| N-formyl-α-D-glucosaminylated maltopentaoside | Non-reducing end | Glycoscience, potential as drug candidate | [67] |
| 2-amino-2-deoxy-α-D-glucopyranosylated maltooligosaccharides | Non-reducing end | Glycoscience | [68] |
| Caproyl maltooligosaccharides | 6II position: 6th carbon of second glucose unit from reducing end | Biodegradable detergents, fine chemicals in cosmetics, and pharmaceutical industry | [69] |
| Galactosyl maltooligosaccharidonolactone | Both non-reducing and reducing end | Substrate analogue inhibitors of mammalian α-amylase | [70] |
| Quercetin-maltooligosaccharides | Reducing end | Food additive or cosmetic ingredient | [71] |
| 2-deoxygluco-maltooligosaccharides | Reducing end | Tracing of the intestinal location of starch | [72] |
| Carboxymethyl derivatives of p-nitrophenyl-α-maltopentaoside | Both non-reducing and reducing end | Activity assay of α-amylases coupled with glucoamylase and α-D-glucosidase | [73] |
| Phosphorylated maltooligosaccharides | Non-reducing end- | Monitoring of transport mechanisms in E. faecalis; in cosmetics as agents for reduction of appearance and visibility of skin pores | [74,75] |
| Enzyme Used | Microbial Source | Type of Reaction | Substrate | Optimal Conditions (Temp. °C, pH) | Predominant Product | Ref. |
|---|---|---|---|---|---|---|
| α-Amylase (EC 3.2.1.1) (Termamyl®) | - | Hydrolysis | Starch | 40, 5.0 | G5 | [82] |
| α-Amylase (EC 3.2.1.1) | Brevibacterium sp. | Hydrolysis | Starch | - | G1–G3 | [83] |
| Chromohalobacter sp. TVSP 101 | 37, 9.0 | G1–G4 | [84] | |||
| Saccharophyla sp. A9 | - | G1–G3 | [85] | |||
| Bacillus megaterium VUMB109 | 93, (-) | G5/G3 | [86] | |||
| Geobacillus stearothermophilus L07 | 70, 6.0 | G6/G5 | [87] | |||
| Bacillus mojavensis A21 | 80, 6.5 | G3/G5/G6 | [88] | |||
| Cryptococcus sp. S-2 | 50, 6.0 | G1–G4 | [89] | |||
| Scytalidium thermophilum 15.1 | 60, 6.0 | G3–G5 | [90] | |||
| Aspergillus oryzae | Transglycosylation | Fluorinated α-D-maltosyl | - | G2–G4 | [77] | |
| MFAse (EC 3.2.1.-) | Bacillus koreensis HL12 | Hydrolysis | Starch | 40, 7.0 | G2–G4 | [7] |
| Brachybacterium sp. LB25 | 35, 6–7.5 | G3 | [91] | |||
| Bacillus circulans GRS313 | 48, 4.9 | G3/G5 | [92] | |||
| Bacillus subtilis KCC103 | 65–70, 6–7 | G1–G7 | [93] | |||
| Bacillus subtilis SDP1 | 37, 7.0 | G2/G3/G5 | [94] | |||
| Bacillus subtilis US116 | 65, 6.0 | G5/G6/G7 | [95] | |||
| G3-Amy (EC 3.2.1.116) | Streptomyces avermitilis NBRC 14893 | Hydrolysis | Starch | -, 6.5 | G3 | [96] |
| Thermobifida fusca NTU22 | 60, 7.0 | [97] | ||||
| Fusicoccum sp. BCC4124 | 70, 7.0 | [98] | ||||
| Microbulbifer thermotolerans DAU221 | 50, 6.0 | [99] | ||||
| Kitasatospora sp. MK-1785 | 55, 6.5 | [100] | ||||
| Bacillus subtilis G3 | 50, 6–7 | [101] | ||||
| Natronococcus sp. Ah-36 | 55, 8.7 | [102] | ||||
| G4-Amy (EC 3.2.1.60) | Bacillus halodurans MS 2-5 | Hydrolysis | Starch | 60–65, 10.5–11.0 | G4 | [103] |
| Pseudomonas stutzeri AS22 | 60, 8.0 | [104] | ||||
| Marinobacter sp. EMB8 | 45, 7.0 | G3/G4 | [105] | |||
| Bacillus sp. GM8901 | 60, 11–12 | G4 | [106] | |||
| Chloroflexus aurantiacus J-10-F1 | 71, 7.5 | G3/G4 | [107] | |||
| Pseudomonas saccharophila IAM1504 | - | G4 | [108] | |||
| Pseudomonas sp. IMD 353 | 50, 7.0 | [109] | ||||
| Pseudomonas sp. MS300 | 40, 6.8–8.9 | [110] | ||||
| Pseudomonas stutzeri MO-19 | 50, 7.0 | [111] | ||||
| Pseudomonas saccharophila STB07 | - | [112] | ||||
| G5-Amy (EC 3.2.1.x) | Bacillus sp. JAMB-204 | Hydrolysis | Starch | 60, 6.5 | G5 | [113] |
| Pseudomonas sp. KO-8940 | - | G5 | [114] | |||
| Bacillus licheniformis NCIB 6346 | 70–90, 7.0 | G5 | [115] | |||
| Bacillus stearothermophilus | 60, 5.5 | G5/G6 | [116] | |||
| G6-Amy (EC 3.2.1.98) | Corallococcus sp. EGB | Hydrolysis | Starch | 50, 7.0 | G6 | [4] |
| Aerobacter aerogenes | 45, 7.0 | G6 | [117] | |||
| Bacillus halodurans LBK34 | 60, 10.5–11.5 | G6 | [118] | |||
| Bacillus sp. 707 | 45, 8.8 | G6 | [119] | |||
| Bacillus sp. H-167 | - | G6 | [120] | |||
| Klebsiella pneumonia IFO-3321 | - | G6 | [121] | |||
| Bacillus stearothermophilus US100 | 82, 5.6 | G6 | [122] | |||
| CGTase (EC 2.4.1.19) | Bacillus circulans DF 9R | Transglycosylation | Glucose/maltose | - | ≤G10 | [123] |
| Amylosucrase (EC 2.4.1.4) and α-amylase (EC 3.2.1.1) | Cellulomonas carboniz and Pseudomonas mendocina | Transglycosylation and hydrolysis | Sucrose | 40, 7.0 and 55, 9.0 | G3/G4 | [124] |
| Immobilized Biocatalyst | Immobilization Technique | Source of Biocatalyst | Support | Optimal Conditions (Temp. °C, pH) | Activity of Immobilized Enzyme (U/g Support) | Reusability Studies (% of Initial Activity/Cycles) | Product Distribution | Ref. |
|---|---|---|---|---|---|---|---|---|
| α-Amylase | Covalent attachment | Aspergillus oryzae | Corn grits | - | 502 | - | G1 (8.5%) | [163] |
| G2 (66.9%) | ||||||||
| G3 (18.8%) | ||||||||
| G4 (2.0%) | ||||||||
| Porous silica (a) | - | 950 | - | G1 (20.6%) | ||||
| G2 (68.5%) | ||||||||
| G3 (3.4%) | ||||||||
| G4 (4.8%) | ||||||||
| Bacillus alcalophilus | Iron-oxid Magnetic Nanoparticles | -, 8 | - | 71%/10 | - | [164] | ||
| Bacillus amyloliquefaciens | Polyaniline Silver Nanoparticles | 60, 6.0 | - | >80%/10 | - | [165] | ||
| Bacillus licheniformis | Poly(HEMA-GMA-1-3) membranes | 60, 6.5 | 390 | - | - | [166] | ||
| PVA-FEP (PVA-coated poly(tetrafluoroethylenehexafluoropropoylene), CDI method (b) | -, 8.4 | 525 | 97%/4 | - | [167] | |||
| Sepharose 4B, CNBr method | -, 8.5 | 1748 | 85%/4 | - | ||||
| Bacillus sp. | Zirconium dynamic membrane, GA method | 41, 5.5 | 59.8 | - | G2, G3, G4 | [168] | ||
| Cryptococcus flavus | Glass tube | 50, 4.5 | - | 47%/10 | - | [169] | ||
| Saccharomyces cerevisiae | Calix[4]arene | 60, 7.0 | - | 62%/10 | - | [170] | ||
| Malt | Chitosan-Fe3O4 CSM | 35, 7.0 | - | 50%/10 | - | [171] | ||
| Ionic Attachment | Chitosan-ZnO CSZ | 35, 6.0 | - | 60%/10 | - | |||
| Bacillus amyloliquifaciens TSWK1-1 | DEAE cellulose | 60, 5.5 | 2186 | 96%/20 | - | [172] | ||
| Covalent attachment | Gelatin | 60, 5.5 | 1771 | 83%/20 | - | |||
| Entrapment | Polyacrylamide | 60, 5.5 | 1563 | 65%/20 | - | |||
| Agar | 60, 5.5 | 1600 | 71%/20 | - | ||||
| Bacillus circulans GRS 313 | Calcium alginate beads | 57, 4.9 | 25.6 | 85%/7 | - | [173] | ||
| Adsorption | Bacillus subtilis | Alumina powder | -, 6.0 | - | - | - | [174] | |
| CLEAs/crosslinking agent | ||||||||
| - | Aspergillus fumigatus | CL GA–1.5% (c) (v/v) | 60, 7.0 | - | 13%/10 | - | [175] | |
| Bacillus licheniformis | CL/BSA + GA | 95, 5.5 | - | 76%/10 | - | [176] | ||
| Bacillus subtilis | CL/Starch | 55, 5.5–7.0 | - | >75%/10 | - | [177] | ||
| CL/BSA | 50, 4.5–7.0 | - | >80%/10 | - | ||||
| CL GA/0.2% (c) (v/v) | 55, 5.5 | - | >70%/10 | - | ||||
| Bacillus sp. | CL GA–0.37% (c) (v/v) | 50, 6.0 | - | 25%/6 | - | [178] | ||
| CL on magnetic nanoparticles GA–0.37% (c) (v/v) | 60, 6.0 | - | 100%/6 | - | ||||
| - | CL/Agar | - | - | 70%/5 | - | [179] | ||
| CL/Chitosan | - | - | 74%/5 | - | ||||
| C L/Dextran | - | - | 79%/5 | - | ||||
| CL/Gum Arabic | - | - | 68%/5 | - | ||||
| CL/GA | - | - | 63%/5 | G4 | ||||
| G4-Amylase + pullulanase | Physical adsorption | G4-Amylase (G4A): Pseudomonas stutzeri NRRL B3389 mutant, pullulanase (P): Klebsiella pneumoniae | Chitosan Beads–Chitopearl BCW Series | 60 (G4A)/55 (P), 7.0 (G4A)/6.0 (P) | 307 (G4A)/84.9 (P) | - | G4 | [180,181] |
| Duolite S-762 | - | 271 (G4A)/74.9 (P) | - | |||||
| Diaion HP-50 | - | 221 (G4A)/44.2 (P) | - | |||||
| G4-Amylase | Pseudomonas stutzeri NRRL B3389 mutant | 55, - | 251 | - | G4 (45%, in later stages of reaction also G1-G3) | [162] | ||
| G6-amylase | Encapsulation by radiocopolymerization | Aerobacter aerogenes UV-mutant | polymer of acrylamide + NN’-methylene bis acrylamide (AA+Bis), calcium acrylate (ACa), sodium acrylate (ANa) | 53, 7.0 | - | 100%/20 | G6 (in later stages of reaction also G1-G5 | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bláhová, M.; Štefuca, V.; Hronská, H.; Rosenberg, M. Maltooligosaccharides: Properties, Production and Applications. Molecules 2023, 28, 3281. https://doi.org/10.3390/molecules28073281
Bláhová M, Štefuca V, Hronská H, Rosenberg M. Maltooligosaccharides: Properties, Production and Applications. Molecules. 2023; 28(7):3281. https://doi.org/10.3390/molecules28073281
Chicago/Turabian StyleBláhová, Mária, Vladimír Štefuca, Helena Hronská, and Michal Rosenberg. 2023. "Maltooligosaccharides: Properties, Production and Applications" Molecules 28, no. 7: 3281. https://doi.org/10.3390/molecules28073281