The SWGEDWGEIW from Soybean Peptides Reduces Insulin Resistance in 3T3-L1 Adipocytes by Activating p-Akt/GLUT4 Signaling Pathway
Abstract
:1. Introduction
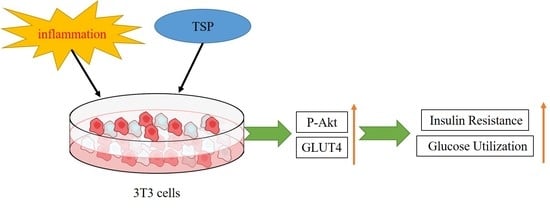
2. Results and Discussion
2.1. Effect of TSP on Insulin Resistance of 3T3-L1 Adipocytes
2.2. Effect of TSP on the Expression Level of Sugar Absorption-Related Proteins in 3T3-L1 Adipocytes
2.3. Analysis of TSP Improving Glucose and Lipid Metabolism in Adipocytes by Activating the p-Akt/GLUT4 Signaling Pathway
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Inflammation Induction Medium
4.3. Induction, Differentiation, and Treatment of 3T3-L1 Preadipocytes
4.4. Cell Viability Detection
4.5. PI3K Inhibitor Interference Treatment
4.6. Detection of Glucose Uptake and Lipid Decomposition in Adipocytes
4.7. Oil Red O Staining
4.8. Western Blot Experiment
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab.-Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Fasshauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2019, 9, 1–58. [Google Scholar]
- Castoldi, A.; de Souza, C.N.; Saraiva Camara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2016, 6, 637. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A Novel Regulator of Macrophage Activation miR-223 in Obesity-Associated Adipose Tissue Inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [Green Version]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Engin, A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation. Obes. Lipotoxicity 2017, 960, 221–245. [Google Scholar]
- Gupta, A.P.; Garg, R.; Singh, P.; Goand, U.K.; Syed, A.A.; Valicherla, G.R.; Riyazuddin, M.; Mugale, M.N.; Gayen, J.R. Pancreastatin inhibitor PSTi8 protects the obesity associated skeletal muscle insulin resistance in diet induced streptozotocin-treated diabetic mice. Eur. J. Pharmacol. 2020, 88, 173–204. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 2006, 84, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Aroor, A.R.; Mandavia, C.H.; Sowers, J.R. Insulin Resistance and Heart Failure: Molecular Mechanisms. Heart Fail. Clin. 2012, 8, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Kundu, R.; Dasgupta, S.; Bhattacharya, S. Mechanism of Lipid Induced Insulin Resistance: An Overview. Endocrinol. Metab. 2012, 27, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, B.; Metzendorf, M.-I.; Richter, B. (Ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2021, 3, 796–812. [Google Scholar] [CrossRef]
- Tiwaskar, M.; Deb, P.; Khadgawat, R.; Sreenivasamurthy, L.; Deka, N.; Karthik, B.; Karuppan, A.; Bhattacharjee, D.; Mohan, V. Pre-Basal Insulin Analog Era: Paving the Way for Modern Day Basal Insulins. J. Assoc. Physicians India 2020, 68, 9–12. [Google Scholar]
- Guan, H.-P.; Chen, G. Factors Affecting Insulin-Regulated Hepatic Gene Expression. In Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus; Tao, Y.X., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 121, pp. 165–215. [Google Scholar]
- Heinemann, L.; Braune, K.; Carter, A.; Zayani, A.; Kramer, L.A. Insulin Storage: A Critical Reappraisal. J. Diabetes Sci. Technol. 2021, 15, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Tiganis, T. PTP1B and TCPTP—Nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J. 2013, 280, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.S.; Medeiros, A.F.; Piuvezam, G.; Medeiros, G.C.B.S.; Maciel, B.L.L.; Morais, A.H.A. Insulin-Like Proteins in Plant Sources: A Systematic Review. Diabetes Metab. Syndr. Obes. 2020, 13, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Huang, H.Q.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two Peptides from Soy beta-Conglycinin Induce a Hypocholesterolemic Effect in HepG2 Cells by a Statin-Like Mechanism: Comparative in Vitro and in Silico Modeling Studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Losso, J.N. The biochemical and functional food properties of the Bowman-Birk inhibitor. Crit. Rev. Food Sci. Nutr. 2008, 48, 94–118. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef] [Green Version]
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. Potential Health Benefits of Lunasin: A Multifaceted Soy-Derived Bioactive Peptide. J. Food Sci. 2015, 80, 485–494. [Google Scholar] [CrossRef]
- Yoshikawa, M. Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides 2015, 72, 208–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, C.; Gleddie, S.; Xiao, C.-W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [Green Version]
- Tsou, M.J.; Kao, F.J.; Lu, H.C.; Kao, H.C.; Chiang, W.D. Purification and identification of lipolysis-stimulating peptides derived from enzymatic hydrolysis of soy protein. Food Chem. 2013, 138, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Li, H.; Liu, M.; Ying, Z.; Zhang, J.; Liu, X. Soybean protein-derived peptides inhibit inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPK-JNK and NF-kappa B activation. J. Food Biochem. 2020, 44, 1321–1340. [Google Scholar] [CrossRef] [PubMed]
- Guofu, Y.; Ud Din, J.; Fen, Z.; Xinqi, L. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar]
- Yi, G.; Li, H.; Li, Y.; Zhao, F.; Ying, Z.; Liu, M.; Zhang, J.; Liu, X.J.F.S. Nutrition, The protective effect of soybean protein-derived peptides on apoptosis via the activation of PI3K-AKT and inhibition on apoptosis pathway. Food Sci. Nutr. 2020, 8, 4591–4600. [Google Scholar] [CrossRef]
- Yi, G.; Safdar, B.; Zhang, Y.; Li, Y.; Liu, X. A study of the mechanism of small-molecule soybean-protein-derived peptide supplement to promote sleep in a mouse model. RSC Adv. 2020, 10, 11264–11273. [Google Scholar] [CrossRef]
- Yi, G.; Zhou, M.; Du, Q.; Yang, S.; Zhu, Y.; Dong, Y.; Liu, Y.; Li, H.; Li, Y.; Liu, X.J.M. The SWGEDWGEIW from Soybean Peptides Reduce Oxidative Damage-Mediated Apoptosis in PC-12 Cells by Activating SIRT3/FOXO3a Signaling Pathway. Molecules 2022, 27, 7610. [Google Scholar] [CrossRef]
- Chirivi, M.; Rendon, C.J.; Myers, M.N.; Prom, C.M.; Roy, S.; Sen, A.; Lock, A.L.; Contreras, G.A. Lipopolysaccharide induces lipolysis and insulin resistance in adipose tissue from dairy cows. J. Dairy Sci. 2022, 105, 842–855. [Google Scholar] [CrossRef]
- Raje, V.; Ahern, K.W.; Martinez, B.A.; Howell, N.L.; Oenarto, V.; Granade, M.E.; Kim, J.W.; Tundup, S.; Bottermann, K.; Gödecke, A. Adipocyte lipolysis drives acute stress-induced insulin resistance. Sci. Rep. 2020, 10, 18166. [Google Scholar] [CrossRef]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, X.; Zhuang, L.; Liu, X.; Zhao, H.; Shan, Y.; Liu, Z.; Li, F.; Wang, Y.; Fang, J. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways. Regul. Toxicol. Pharmacol. 2020, 110, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.J.; Gould, G.W. Insulin stimulated GLUT4 translocation–Size is not everything! Curr. Opin. Cell Biol. 2020, 65, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and insulin resistance: A review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-P.; Huang, S.-S.; Deng, J.-Y.; Hung, L.-M. Impairment of insulin-stimulated Akt/GLUT4 signaling is associated with cardiac contractile dysfunction and aggravates I/R injury in STZ-diabetic rats. J. Biomed. Sci. 2009, 16, 77. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of PPARγ and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 2243–2255. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Guo, Q.; Xu, L.; Santhanam, R.K.; Gao, X.; Chen, Y.; Wang, C.; Panichayupakaranant, P.; Chen, H. Anthocyanins from dietary black soybean potentiate glucose uptake in L6 rat skeletal muscle cells via up-regulating phosphorylated Akt and GLUT4. J. Funct. Foods 2019, 52, 663–669. [Google Scholar] [CrossRef]
- Arjunan, S.; Thangaiyan, R.; Balaraman, D. Biochanin A, a soy isoflavone, diminishes insulin resistance by modulating insulin-signalling pathway in high-fat diet-induced diabetic mice: Biochanin A diminishes insulin resistance in diabetic mice. Arch. Physiol. Biochem. 2020, 2, 1–7. [Google Scholar] [CrossRef]
- Ghadimi, D.; Hemmati, M.; Karimi, N.; Khadive, T. Soy Isoflavone Genistein Is a Potential Agent for Metabolic Syndrome Treatment: A Narrative Review. J. Adv. Med. Biomed. Res. 2020, 28, 64–75. [Google Scholar] [CrossRef]
- Das, D.; Afzal, N.U.; Wann, S.B.; Kalita, J.; Manna, P. A ~24 kDa protein isolated from protein isolates of Hawaijar, popular fermented soy food of North-East India exhibited promising antidiabetic potential via stimulating PI3K/AKT/GLUT4 signaling pathway of muscle glucose metabolism. Int. J. Biol. Macromol. 2023, 224, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.J.; Kim, C.S.; Choi, M.S.; Park, T.; Sung, M.K.; Yun, J.W.; Yoo, H.; Mine, Y.; Yu, R. The Soy Peptide Phe-Leu-Val Reduces TNFα-Induced Inflammatory Response and Insulin Resistance in Adipocytes. J. Med. Food 2016, 19, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R.J.F. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Yamagata, K.; Fujiyama, S.; Matsumoto, T.; Koshida, I.; Yoshimura, K.; Mihara, M.; Naitou, M.; Endoh, H.; Nakamura, T.; et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat. Cell Biol. 2007, 9, 604–613. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.; Li, P.; Li, L.J.O.R.; Practice, C. A novel adipokine WISP1 attenuates lipopolysaccharide-induced cell injury in 3T3-L1 adipocytes by regulating the PI3K/Akt pathway. Obes. Res. Clin. Pract. 2022, 16, 122–129. [Google Scholar] [CrossRef]
- Manaharan, T.; Ming, C.H.; Palanisamy Uma, D. Syzygium aqueum leaf extract and its bioactive compounds enhances pre-adipocyte differentiation and 2-NBDG uptake in 3T3-L1 cells. Food Chem. 2013, 136, 354–363. [Google Scholar] [CrossRef]
- Deutsch, M.J.; Schriever, S.C.; Roscher, A.A.; Ensenauer, R. Digital image analysis approach for lipid droplet size quantitation of Oil Red O-stained cultured cells. Anal. Biochem. 2014, 445, 87–89. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, G.; Sang, X.; Zhu, Y.; Zhou, D.; Yang, S.; Huo, Y.; Liu, Y.; Safdar, B.; Bu, X. The SWGEDWGEIW from Soybean Peptides Reduces Insulin Resistance in 3T3-L1 Adipocytes by Activating p-Akt/GLUT4 Signaling Pathway. Molecules 2023, 28, 3001. https://doi.org/10.3390/molecules28073001
Yi G, Sang X, Zhu Y, Zhou D, Yang S, Huo Y, Liu Y, Safdar B, Bu X. The SWGEDWGEIW from Soybean Peptides Reduces Insulin Resistance in 3T3-L1 Adipocytes by Activating p-Akt/GLUT4 Signaling Pathway. Molecules. 2023; 28(7):3001. https://doi.org/10.3390/molecules28073001
Chicago/Turabian StyleYi, Guofu, Xia Sang, Yuxia Zhu, Di Zhou, Shuibing Yang, Yue Huo, Yang Liu, Bushra Safdar, and Xianyong Bu. 2023. "The SWGEDWGEIW from Soybean Peptides Reduces Insulin Resistance in 3T3-L1 Adipocytes by Activating p-Akt/GLUT4 Signaling Pathway" Molecules 28, no. 7: 3001. https://doi.org/10.3390/molecules28073001