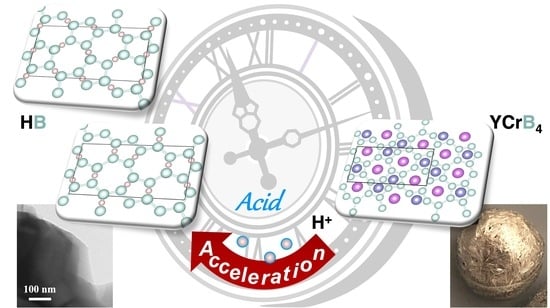
Accelerated Synthesis of Borophane (HB) Sheets through HCl-Assisted Ion-Exchange Reaction with YCrB4
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis Process
2.2. Enhancement of the HB Product by the Acid
2.3. Analysis of the Precipitate Residue after the Reaction
3. Materials and Methods
3.1. Synthesis Methods
3.2. Characterization Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.; Geim, A.; Morozov, S.; Jiang, D.; Katsnelson, M.; Grigorieva, I.; Dubonos, S.; Firsov, A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neto, A.; Guinea, F.; Peres, N.; Novoselov, K.; Geim, K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, I. Monatomic Two-Dimensional Layers: Modern Experimental Approaches for Structure, Properties, and Industrial Use; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chhowalla, M.; Shin, H.; Eda, G.; Li, L.; Loh, L.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Nayak, A.; Sun, Y.; Schmidt, M.; Nicklas, M.; Leermakers, I.; Zeitler, U.; Skourski, Y.; Wosnitza, J.; Liu, Z.; et al. Extremely large magnetoresistance and ultrahigh mobility in the topological Weyl semimetal candidate NbP. Nat. Phys. 2015, 11, 645. [Google Scholar] [CrossRef]
- Nair, R.; Blake, P.; Grigorenko, A.; Novoselov, K.; Booth, T.; Stauber, T.; Peres, N.; Geim, A. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, I.; Wu, K. (Eds.) 2D Boron: Boraphene, Borophene, Boronene, 1st ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Feng, B.; Zhang, J.; Ito, S.; Arita, M.; Cheng, C.; Chen, L.; Wu, K.; Komori, F.; Sugino, O.; Miyamoto, K.; et al. Discovery of 2D Anisotropic Dirac Cones. Adv. Mater. 2018, 30, 1704025. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Sugino, O.; Liu, R.Y.; Zhang, J.; Yukawa, R.; Kawamura, M.; Iimori, T.; Kim, H.; Hasegawa, Y.; Li, H.; et al. Dirac Fermions in Borophene. Phys. Rev. Lett. 2017, 118, 096401. [Google Scholar] [CrossRef] [Green Version]
- Ando, Y.; Zhang, X.; Tsujikawa, Y.; Sato, Y.; Horio, M.; Haruyama, J.; Sugino, O.; Kondo, T.; Matsuda, I. Homotopic Analysis of Quantum States in Two-Dimensional Polymorphs by a Herringbone Lattice Model. Phys. Rev. B 2022, 106, 195106. [Google Scholar] [CrossRef]
- Tsujikawa, Y.; Horio, M.; Zhang, X.; Senoo, T.; Nakashima, T.; Ando, Y.; Ozaki, T.; Mochizuki, I.; Wada, K.; Hyodo, T.; et al. Structural and electronic evidence of boron atomic chains. Phys. Rev. B 2022, 106, 205406. [Google Scholar] [CrossRef]
- Lopez-Bezanilla, A.; Littlewood, P.B. Electronic Properties of 8-Pmmn Borophene. Phys. Rev. B 2016, 93, 241405. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Li, Q.-Z.; Yan, X.-W.; Wang, J. Prediction of Phonon-Mediated Superconductivity in Borophene. Phys. Rev. B 2017, 95, 024505. [Google Scholar] [CrossRef] [Green Version]
- Feng, B.; Zhang, J.; Liu, R.-Y.; Iimori, T.; Lian, C.; Li, H.; Chen, L.; Wu, K.; Meng, S.; Komori, F.; et al. Direct Evidence of Metallic Bands in a Monolayer Boron Sheet. Phys. Rev. B 2016, 94, 041408. [Google Scholar] [CrossRef] [Green Version]
- Mannix, A.; Zhou, X.; Kiraly, B.; Wood, J.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.; Santiago, U.; Guest, J.; et al. Synthesis of Borophenes: Anisotropic, Two-dimensional Boron Polymorphs. Science 2015, 350, 15131516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, H.; Fujita, T.; Cuong, N.T.; Tominaka, S.; Miyauchi, M.; Iimura, S.; Hirata, A.; Umezawa, N.; Okada, S.; Nishibori, E.; et al. Formation and Characterization of Hydrogen Boride Sheets Derived from MgB2 by Cation Exchange. J. Am. Chem. Soc. 2017, 139, 13761–13769. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, I.; Cuong, N.T.; Moura, C.A.S.; Cameau, M.; Ishibiki, R.; Fujino, A.; Okada, S.; Yamamoto, A.; Araki, M.; Ito, S.; et al. Semimetallicity of Free-standing Hydroenated Monolayer Boron from MgB2. Phys. Rev. Mat. 2019, 3, 024004. [Google Scholar]
- Niibe, M.; Cameau, M.; Cuong, N.T.; Sunday, O.I.; Zhang, X.; Tsujikawa, Y.; Okada, S.; Yubuta, K.; Kondo, T.; Matsuda, I. Electronic Structure of a Borophene Layer in Rare-earth Aluminum/Chromium Boride and its Hydrogenated Derivative Borophane. Phys. Rev. Mat. 2020, 5, 084007. [Google Scholar] [CrossRef]
- Tateishi, I.; Zhang, X.; Matsuda, I. Electronic Structures of Polymorphic Layers of Borophane. Molecules 2022, 27, 1808. [Google Scholar] [CrossRef]
- Zhang, X.; Tsujikawa, Y.; Tateishi, I.; Niibe, M.; Wada, T.; Horio, M.; Hikichi, M.; Ando, Y.; Yubuta, K.; Kondo, T.; et al. Electronic Topological Transition of 2D Boron by the Ion Exchange Reaction. J. Phys. Chem. C. 2022, 126, 12802–12808. [Google Scholar] [CrossRef]
- Cuong, N.T.; Tateishi, I.; Cameau, M.; Niibe, M.; Umezawa, N.; Slater, B.; Yubuta, K.; Kondo, T.; Ogata, M.; Okada, S.; et al. Topological Dirac Nodal Loops in Nonsym-morphic Hydrogenated Monolayer Boron. Phys. Rev. B 2020, 101, 195412. [Google Scholar] [CrossRef]
- Kawamura, R.; Yamaguchi, A.; Shimada, C.; Ishibiki, R.; Fujita, T.; Kondo, T.; Miyauchi, M. Acid Assisted Synthesis of HB Sheets Through Exfoliation of MgB2 Bulk in Organic Media. Chem. Lett. 2020, 49, 1194–1196. [Google Scholar] [CrossRef]
- Tominaka, S.; Ishibiki, R.; Fujino, A.; Kawakami, K.; Ohara, K.; Masuda, T.; Matsuda, I.; Hosono, H.; Kondo, T. Geometrical Frustration of BH Bonds in Layered Hydrogen Borides Accessible by Soft Chemistry. Chem 2020, 6, 406. [Google Scholar] [CrossRef]
- Rojas, K.I.M.; Cuong, N.T.; Nishino, H.; Ishibiki, R.; Ito, S.; Miyauchi, Y.; Fujimoto, Y.; Tominaka, S.; Okada, S.; Hosono, H.; et al. Chemical Stability of Hydrogen Boride Nanosheets in Water. Commun. Mater. 2021, 2, 81. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.Y.; Yu, C.C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Hao, J.; Sheng, L.; Liu, B.; Wu, Z. Ultrastable Crystalline Semiconducting Hydrogenated Borophene. Angew. Chem. Int. Ed. 2020, 59, 10819–10825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tian, X.; Sheng, S.; Ma, C.; Chen, L.; Feng, B.; Zhang, Y.; Chen, L.; Zhao, J.; Wu, K. Vibrational Property of α-Borophene Determined by Tip-Enhanced Raman Spectroscopy. Molecules 2022, 27, 834. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.; Burkholder, T.R. Infrared Spectra of Molecular B(OH)3 and HOBO in Solid Argon. J. Chem. Phys. 1992, 97, 7203. [Google Scholar] [CrossRef]
- Kawamura, R.; Cuong, N.T.; Fujita, T.; Ishibiki, R.; Hirabayashi, T.; Yamaguchi, A.; Matsuda, I.; Okada, S.; Kondo, T.; Miyauchi, M. Photoinduced Hydrogen Release from Hydrogen Boride Sheets. Nat. Commun. 2019, 10, 4880. [Google Scholar] [CrossRef] [Green Version]
- Uwamino, Y.; Tsuge, A.; Ishizuka, T.; Yamatera, H. X-ray Photoelectron Spectroscopy of Rare Earth Halides. Bull. Chem. Soc. Jpn. 1986, 59, 2263–2267. [Google Scholar] [CrossRef] [Green Version]
- Sleigh, C.; Pijpers, A.P.; Jaspers, A.; Coussens, B.; Meier, R.J. On the Determination of Atomic Charge via ESCA Including Application to Organometallics. J. Electron Spectrosc. Relat. Phenom. 1996, 77, 41–57. [Google Scholar] [CrossRef]











| Label | 3 h | 1 day | 3 days | Post-Filtration | Reference (Vibration Mode) |
|---|---|---|---|---|---|
| A | 634 | 631 | 635 | 632 | B-B skeletal [26,27] |
| B | 1652 | 1654 | 1645 | 1639 | B-H-B linkage [16] |
| C | 3232 | 3524 | 3014 | 3429 | O-H stretching [28] |
| D | - | - | 1050 | - | H-O-B deforming [28] |
| E | - | - | 1540 | - | B-O stretching [29] |
| Label | 3 h | 1 day | 3 days | Post-Filtration | Reference (Vibration Mode) |
|---|---|---|---|---|---|
| A’ | 639 | - | - | - | B-B skeletal [26] |
| B’ | 1627 | 1642 | - | - | B-H-B linkage [16] |
| C’ | 3540 | 3550 | 3500 | - | O-H stretching [29] |
| D’ | - | 1050 | - | - | B-H stretching [22,29] |
| E’ | - | 1512 | - | - | B-O stretching [28] |
| F’ | - | 2364 | - | - | B-H stretching [23] |
| C” | - | - | 3225 | - | O-H stretching [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hikichi, M.; Iimori, T.; Tsujikawa, Y.; Yuan, M.; Horio, M.; Yubuta, K.; Komori, F.; Miyauchi, M.; Kondo, T.; et al. Accelerated Synthesis of Borophane (HB) Sheets through HCl-Assisted Ion-Exchange Reaction with YCrB4. Molecules 2023, 28, 2985. https://doi.org/10.3390/molecules28072985
Zhang X, Hikichi M, Iimori T, Tsujikawa Y, Yuan M, Horio M, Yubuta K, Komori F, Miyauchi M, Kondo T, et al. Accelerated Synthesis of Borophane (HB) Sheets through HCl-Assisted Ion-Exchange Reaction with YCrB4. Molecules. 2023; 28(7):2985. https://doi.org/10.3390/molecules28072985
Chicago/Turabian StyleZhang, Xiaoni, Miwa Hikichi, Takushi Iimori, Yuki Tsujikawa, Mei Yuan, Masafumi Horio, Kunio Yubuta, Fumio Komori, Masahiro Miyauchi, Takahiro Kondo, and et al. 2023. "Accelerated Synthesis of Borophane (HB) Sheets through HCl-Assisted Ion-Exchange Reaction with YCrB4" Molecules 28, no. 7: 2985. https://doi.org/10.3390/molecules28072985