Variation of Aroma Components of Pasteurized Yogurt with Different Process Combination before and after Aging by DHS/GC-O-MS
Abstract
:1. Introduction
2. Results and Discussion
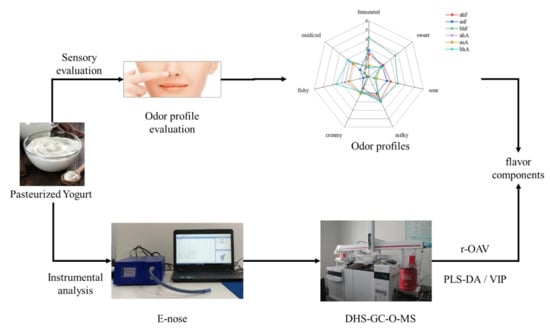
2.1. Establishment of Overall Odor Profile
2.2. Volatiles in Pasteurized Yogurt Analyzed via E-Nose
2.3. Volatile Compounds in Pasteurized Yogurt Analyzed via GC-MS
2.4. Odor-Active Compounds in Pasteurized Yogurt Analyzed via GC-O-MS
2.5. The Contribution Degree of Odor-Active Compounds to the Overall Odor Profile of Pasteurized Yogurt Evaluated by r-OAV
2.6. Differential Compounds of Pasteurized Yogurt before and after Aging Identified by PLS-DA
3. Materials and Methods
3.1. Samples
3.2. Reagents and Chemicals
3.3. Establishment of Odor Profiles
3.4. Volatiles Analysis by Electronic Nose (E-Nose)
3.5. Extraction of Volatile Compounds by Dynamic Headspace Sampling (DHS)
3.6. Gas Chromatography–Mass Spectrometry (GC-MS)/Gas Chromatography–Olfactometry (GC-O) Analysis
3.7. Identification of Volatile Compounds and Odor-Active Compounds
3.8. Quantitation of Compounds
3.9. Calculation of Relative Odor Activity Value (r-OAV)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- O’Brien, K.V.; Aryana, K.J.; Prinyawiwatkul, W.; Ordonez, K.C.; Boeneke, C.A. Short communication: The effects of frozen storage on the survival of probiotic microorganisms found in traditionally and commercially manufactured kefir. J. Dairy Sci. 2016, 99, 7043–7048. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Shi, Y.; Zhang, Y.; Yu, H.; Mu, H.; Chen, C. Screening of aroma-producing lactic acid bacteria and their application in improving the aromatic profile of yogurt. J. Food Biochem. 2019, 43. [Google Scholar] [CrossRef]
- Miyaji, K.; Maruyama, H.; Kuwano, Y.; Katakura, Y.; Inoue, H.; Azuma, N. Development of a Rapid and Accurate Prediction Model for Whey Separation in Pasteurized Drinking Yogurt Caused by Long-term Ambient Storage. Food Sci. Technol. Res. 2020, 26, 863–873. [Google Scholar] [CrossRef]
- Ding, R.; Li, M.; Zou, Y.; Wang, Y.; Yan, C.; Zhang, H.; Wu, R.; Wu, J. Effect of normal and strict anaerobic fermentation on physicochemical quality and metabolomics of yogurt. Food Biosci. 2022, 46. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC × GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, J.; Liu, H.; Feng, C.; Shuang, Q. Novel insight into physicochemical and flavor formation in koumiss based on microbial metabolic network. Food Res. Int. 2021, 149. [Google Scholar] [CrossRef]
- Sfakianakis, P.; Tzia, C. Flavour profiling by gas chromatography–mass spectrometry and sensory analysis of yoghurt derived from ultrasonicated and homogenised milk. Int. Dairy J. 2017, 75, 120–128. [Google Scholar] [CrossRef]
- Zhou, T.; Huo, R.; Kwok, L.-Y.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of applying Lactobacillus helveticus H9 as adjunct starter culture in yogurt fermentation and storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Martin, F.; Cachon, R.; Pernin, K.; De Coninck, J.; Gervais, P.; Guichard, E.; Cayot, N. Effect of oxidoreduction potential on aroma biosynthesis by lactic acid bacteria in nonfat yogurt. J. Dairy Sci. 2011, 94, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moineau-Jean, A.; Raymond, Y.; Sabik, H.; Graveline, N.; Champagne, C.P.; Roy, D.; LaPointe, G. Effect of manufacturing processes and storage on aroma compounds and sensory properties of yoghurt. Int. Dairy J. 2020, 105. [Google Scholar] [CrossRef]
- Rychlik, M.; Sax, M.; Schieberle, P. On the role of short-chain free fatty acids for the development of a cheese-like off-note in pasteurized yoghurt. Lwt Food Sci. Technol. 2006, 39, 521–527. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, W.J. Short communication: Formation of oxidized flavor compounds in concentrated milk and distillate during milk concentration. J. Dairy Sci. 2016, 99, 9647–9651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.H.; Zhang, L.W.; Wang, W.J.; Han, X. Differences in particle characteristics and oxidized flavor as affected by heat-related processes of milk powder. J. Dairy Sci. 2013, 96, 4784–4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Ma, H.; Hou, Y.; Li, J.; Zou, T.; Zhang, D.; Wen, R.; Li, H.; Song, H. Characterization of Key Odor-Active Off-Flavor Compounds in Aged Pasteurized Yogurt by Sensory-Directed Flavor Analysis. J. Agric. Food Chem. 2022, 70, 14439–14447. [Google Scholar] [CrossRef]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Liu, C.; Yang, P.; Wang, H.; Song, H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. Lwt 2022, 154. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Effect of High-Pressure−Moderate-Temperature Processing on the Volatile Profile of Milk. J. Agric. Food Chem. 2006, 54, 9184–9192. [Google Scholar] [CrossRef]
- Gathercole, J.; Reis, M.G.; Agnew, M.; Reis, M.M.; Humphrey, R.; Harris, P.; Clerens, S.; Haigh, B.; Dyer, J.M. Molecular modification associated with the heat treatment of bovine milk. Int. Dairy J. 2017, 73, 74–83. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D.; Sun, Y.; Cao, J. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. Eur. J. Lipid Sci. Technol. 2017, 119. [Google Scholar] [CrossRef]
- Zannou, O.; Kelebek, H.; Selli, S. Elucidation of key odorants in Beninese Roselle (Hibiscus sabdariffa L.) infusions prepared by hot and cold brewing. Food Res. Int. 2020, 133. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME–GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef]
- Ott, A.; Hugi, A.; Baumgartner, M.; Chaintreau, A. Sensory Investigation of Yogurt Flavor Perception: Mutual Influence of Volatiles and Acidity. J. Agric. Food Chem. 2000, 48, 441–450. [Google Scholar] [CrossRef]
- Ramos, A.; Jordan, K.N.; Cogan, T.M.; Santos, H. 13 C Nuclear Magnetic Resonance Studies of Citrate and Glucose Cometabolism by Lactococcus lactis. Appl. Environ. Microbiol. 1994, 60, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Reis, M.G.; Harris, P.; Berry, C.; Nguyen, H.; Maclean, P.; Weeks, M. Tracking changes in volatile components and lipids after homogenisation and thermal processing of milk. Int. Dairy J. 2020, 103. [Google Scholar] [CrossRef]
- Lozano, P.R.; Miracle, E.R.; Krause, A.J.; Drake, M.; Cadwallader, K.R. Effect of Cold Storage and Packaging Material on the Major Aroma Components of Sweet Cream Butter. J. Agric. Food Chem. 2007, 55, 7840–7846. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.J. Odour Thresholds-Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Liu, H.; Hui, T.; Zheng, X.; Li, S.; Wei, X.; Li, P.; Zhang, D.; Wang, Z. Characterization of key lipids for binding and generating aroma compounds in roasted mutton by UPLC-ESI-MS/MS and Orbitrap Exploris GC. Food Chem. 2022, 374. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pu, X.; Sun, J.; Shi, X.; Cheng, W.; Wang, B. Effect of Lactobacillus plantarum on functional characteristics and flavor profile of fermented walnut milk. Lwt 2022, 160. [Google Scholar] [CrossRef]
- Zabbia, A.; Buys, E.M.; De Kock, H.L. Undesirable Sulphur and Carbonyl Flavor Compounds in UHT Milk: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 21–30. [Google Scholar] [CrossRef]
- Xu, L.; Mei, X.; Chang, J.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Comparative characterization of key odorants of French fries and oils at the break-in, optimum, and degrading frying stages. Food Chem. 2022, 368. [Google Scholar] [CrossRef]
- Yang, S.; Yan, D.; Zou, Y.; Mu, D.; Li, X.; Shi, H.; Luo, X.; Yang, M.; Yue, X.; Wu, R.; et al. Fermentation temperature affects yogurt quality: A metabolomics study. Food Biosci. 2021, 42. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, H.; Hayaloglu, A.A. Volatiles and sensory characteristics of yogurt manufactured by incorporating basil (Ocimum basilicum L.). Int. J. Food Prop. 2017, 20, S779–S789. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Feng, T.; Sheng, M.; Wang, B.; Wang, Z.; Shan, P.; Zhang, Y.; Ma, H. Characterization of the aroma-active compounds in black soybean sauce, a distinctive soy sauce. Food Chem. 2021, 364. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Feng, T.; Liu, E.; Shan, P.; Zhang, Z.; Liao, L.; Ma, H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: Impacts on aroma and taste. Food Chem. 2021, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, T.; Yang, F.; Cui, X.; Zou, T.; Song, H.; Liu, Y. Characterization of key aroma-active compounds in Hanyuan Zanthoxylum bungeanum by GC-O-MS and switchable GC × GC-O-MS. Food Chem. 2022, 385, 132659. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, J.; Tang, X.; Li, T.; Duan, S. Characterization and identification of pork flavor compounds and their precursors in Chinese indigenous pig breeds by volatile profiling and multivariate analysis. Food Chem. 2022, 385, 132543. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Lin, Y.; Guo, T.; Wang, L.; Granvogl, M.; Xu, Y. Differences of characteristic aroma compounds in Rougui tea leaves with different roasting temperatures analyzed by switchable GC-O-MS and GC × GC-O-MS and sensory evaluation. Food Funct. 2021, 12, 4797–4807. [Google Scholar] [CrossRef]




| No. | Receptor | Specifification |
|---|---|---|
| R1 | W1C | aromatic hydrocarbon |
| R2 | W5S | broad range |
| R3 | W3C | aromatic ammonia |
| R4 | W6S | hydrogen |
| R5 | W5C | arom-aliph |
| R6 | W1S | broad-methane |
| R7 | W1W | sulfur-organic |
| R8 | W2S | broad-alcohol |
| R9 | W2W | sulfur-chlor |
| R10 | W3S | methane-aliph |
| No. | CAS | Compounds | Odor | Identification | RI a | Concentration (μg/g) b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-WAX | DB-5 | ahF | asF | bhF | ahA | asA | bhA | |||||
| 1 | 75-07-0 | Acetaldehyde | grass-like | MS/RI/O | <800 | - | 1.71 ± 0.00145 b | 0.545 ± 0.0237 c | 2.16 ± 0.0357 a | 4.13 ± 0.469 a | 2.28 ± 0.0834 a | 2.99 ± 0.264 a |
| 2 | 431-03-8 | 2,3-Butanedione | pungent buttery | MS/RI/O | 980 | <800 | 2.45 ± 0.0105 b | 3.28 ± 0.0248 a | 3.88 ± 0.136 a | 4.03 ± 0.117 a | 2.75 ± 0.0350 b | 4.63 ± 0.861 a |
| 3 | 600-14-6 | 2,3-Pentanedione | buttery | MS/RI/O | 1053 | <800 | 2.12 ± 0.0507 a | 2.43 ± 0.116 a | 2.84 ± 0.0757 a | - | - | - |
| 4 | 66-25-1 | Hexanal | grass-like | MS/RI/O | 1079 | 803 | 0.423 ± 0.0427 c | 0.124 ± 0.0408 c | 0.125 ± 0.00338 c | 53.6 ± 9.62 ab | 30.3 ± 1.32 b | 64.5 ± 19.8 a |
| 5 | 110-43-0 | 2-Heptanone | sweet | MS/RI/O | 1179 | 888 | 2.56 ± 0.350 b | 2.94 ± 0.391 b | 1.43 ± 0.189 b | 13.1 ± 0.990 a | 3.18 ± 0.631 b | 13.8 ± 3.10 a |
| 6 | 100-42-5 | Styrene | plastic-like | MS/RI/O | 1246 | - | 0.184 ± 0.00218 c | 0.475 ± 0.0538 b | 0.180 ± 0.00937 c | 0.892 ± 0.247 a | 0.880 ± 0.0618 a | 1.44 ± 0.251 a |
| 7 | 513-86-0 | Acetoin | mild creamy | MS/RI/O | 1281 | <800 | 68.1 ± 1.53 c | 76.7 ± 7.03 bc | 93.7 ± 4.60 b | 82.4 ± 2.03 b | 84.6 ± 11.1 b | 138 ± 8.23 a |
| 8 | 821-55-6 | 2-Nonanone | sweet | MS/RI/O | 1381 | - | 0.443 ± 0.0730 b | 0.156 ± 0.00409 c | 0.235 ± 0.0148 bc | 0.569 ± 0.194 ab | 0.384 ± 0.00406 b | 0.913 ± 0.236 a |
| 9 | 2548-87-0 | (E)-2-Octenal | fresh | MS/RI/O | 1420 | - | - | - | - | 0.834 ± 0.246 ab | 0.423 ± 0.0638 b | 0.911 ± 0.174 a |
| 10 | 64-19-7 | Acetic acid | vinegar-like | MS/RI/O | 1461 | <800 | 1.35 ± 0.194 a | 1.79 ± 0.0537 a | 1.63 ± 0.0837 a | 1.23 ± 0.163 a | 2.12 ± 0.331 a | 1.54 ± 0.213 a |
| 11 | 100-52-7 | Benzaldehyde | almond-like | MS/RI/O | 1509 | 956 | 1.65 ± 0.248 c | 1.49 ± 0.0684 c | 1.41 ± 0.213 c | 4.58 ± 0.280 b | 4.83 ± 0.508 b | 12.3 ± 0.830 a |
| 12 | 107-92-6 | Butyric acid | sweaty | MS/RI/O | 1630 | <800 | 8.05 ± 0.772 c | 5.54 ± 1.38 cd | 3.63 ± 0.157 d | 80.0 ± 4.75 b | 59.7 ± 3.96 b | 143 ± 4.18 a |
| 13 | 109-52-4 | Pentanoic acid | sweaty | MS/RI/O | 1734 | 912 | - | - | - | 1.42 ± 0.0243 | - | - |
| 14 | 142-62-1 | Hexanoic acid | cheesy | MS/RI/O | 1851 | 984 | 17.9 ± 2.58 a | 10.3 ± 5.44 ab | 13.4 ± 0.526 a | 9.49 ± 0.327 ab | 8.11 ± 0.966 b | 13.9 ± 3.36 a |
| 15 | 124-07-2 | Octanoic acid | cheesy | MS/RI/O | 2083 | 1174 | 11.8 ± 0.255 a | 9.59 ± 0.0197 b | 9.51 ± 0.00387 b | 10.3 ± 0.824 ab | 7.69 ± 0.371 b | 13.7 ± 0.763 a |
| CAS | Compounds | OT (mg/kg) | r-OAV | |||||
|---|---|---|---|---|---|---|---|---|
| ahF | asF | bhF | ahA | asA | bhA | |||
| 431-03-8 | 2,3-Butanedione | 0.006 | 408 | 547 | 647 | 672 | 458 | 772 |
| 600-14-6 | 2,3-Pentanedione | 0.02 | 106 | 122 | 142 | - | - | - |
| 66-25-1 | Hexanal | 0.005 | 84 | 24 | 24 | 10,726 | 6062 | 12,896 |
| 75-07-0 | Acetaldehyde | 0.063 | 27 | 9 | 34 | 66 | 36 | 47 |
| 110-43-0 | 2-Heptanone | 0.14 | 18 | 21 | 10 | 93 | 23 | 99 |
| 821-55-6 | 2-Nonanone | 0.041 | 10 | 4 | 6 | 14 | 9 | 22 |
| 513-86-0 | Acetoin | 8 | 9 | 10 | 12 | 10 | 11 | 17 |
| 100-52-7 | Benzaldehyde | 0.35 | 5 | 4 | 4 | 13 | 14 | 35 |
| 124-07-2 | Octanoic acid | 3 | 4 | 3 | 3 | 3 | 3 | 5 |
| 100-42-5 | Styrene | 0.065 | 3 | 7 | 3 | 14 | 14 | 22 |
| 107-92-6 | Butanoic acid | 2.4 | 3 | 2 | 2 | 33 | 25 | 60 |
| 142-62-1 | Hexanoic acid | 18 | 1 | 1 | 1 | 1 | <1 | 1 |
| 64-19-7 | Acetic acid | 22 | <1 | <1 | <1 | <1 | <1 | <1 |
| 2548-87-0 | (E)-2-Octenal | 0.003 | - | - | - | 277 | 140 | 303 |
| 109-52-4 | Pentanoic acid | 1.207 | - | - | - | 1 | - | - |
| Sample Type | Process Combination | Abbreviation |
|---|---|---|
| Fresh pasteurized yogurt (F) | Fermentation process a & homogenizer treated (ah) | ahF |
| Fermentation process a & smooth pump treated (as) | asF | |
| Fermentation process b & homogenizer treated (bh) | bhF | |
| Aged pasteurized yogurt (A) | Fermentation process a & homogenizer treated (ah) | ahA |
| Fermentation process a & smooth pump treated (as) | asA | |
| Fermentation process b & homogenizer treated (bh) | bhA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, H.; Zhang, D.; Li, J.; Wen, R.; Ma, H.; Zou, T.; Hou, Y.; Song, H. Variation of Aroma Components of Pasteurized Yogurt with Different Process Combination before and after Aging by DHS/GC-O-MS. Molecules 2023, 28, 1975. https://doi.org/10.3390/molecules28041975
Zhao M, Li H, Zhang D, Li J, Wen R, Ma H, Zou T, Hou Y, Song H. Variation of Aroma Components of Pasteurized Yogurt with Different Process Combination before and after Aging by DHS/GC-O-MS. Molecules. 2023; 28(4):1975. https://doi.org/10.3390/molecules28041975
Chicago/Turabian StyleZhao, Mu, Hongliang Li, Dongjie Zhang, Jie Li, Rong Wen, Hairan Ma, Tingting Zou, Yaqiong Hou, and Huanlu Song. 2023. "Variation of Aroma Components of Pasteurized Yogurt with Different Process Combination before and after Aging by DHS/GC-O-MS" Molecules 28, no. 4: 1975. https://doi.org/10.3390/molecules28041975