Calix[4]arene Derivative for Iodine Capture and Effect on Leaching of Iodine through Packaging
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Tetra(ethoxycarbonyl-methoxy)-4-tert-butylcalix[4]arene
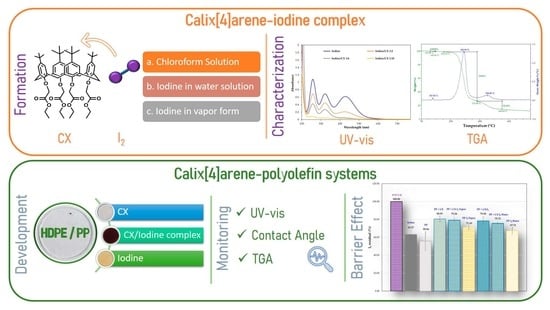
2.2. Formation of CX/Iodine Inclusion Complex
2.2.1. Formation of the Complex in Chloroform Solution
2.2.2. Formation of the Complex by Solid CX and Iodine in Water Solution (Solid–Liquid Method)
2.2.3. Formation of the Complex by Solid CX and Iodine in Vapor form (Solid–Air Method)
2.3. Stability of the CX/Iodine Inclusion Complex
2.4. Calix[4]arene-Polyolefin Systems
2.5. Influence of Calix[4]arene Derivative-Polypropylene Systems on Leaching of Iodine through Packaging from a PVP-I Buffered Solution
3. Materials and Methods
3.1. Materials
3.2. Preparation of CX/Iodine Complex in Chloroform
3.3. Preparation of CX/Iodine Complex from Iodine Water Solution
3.4. Preparation of CX/Iodine Complex from Iodine Vapors
3.5. Preparation of Polyolefin Films
3.6. Embedding of CX/Iodine Complex in HDPE/PP Samples
3.7. Embedding of CX in HDPE/PP Samples
3.8. Loading of Iodine in the CX-Embedded PP Samples
3.9. Nuclear Magnetic Resonance (NMR)
3.10. UV-Vis Spectroscopy
3.11. Thermogravimetric Analysis (TGA)
3.12. Contact Angle Measurements
3.13. Determination of Leaching of Iodine through CX-Loaded PP Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustsche, C.D. Calixarenes Revisited; The Royal Society of Chemistry: Cambridge, UK, 1998. [Google Scholar]
- Mandolini, L.; Ungaro, R. Calixarenes in Action; Imperial College Press: London, UK, 2000. [Google Scholar]
- Bohmer, V. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew. Chem. Int. Ed. Engl. 1995, 34, 713–725. [Google Scholar] [CrossRef]
- Taghvaei-Ganjali, S.; Khosravi, M.; Tahvildari, K.; Naderi, A. Spectroscopic studies of interaction between Iodine and Benzyloxy ether Derivatives of Calix[4]arene. J. Sci. Islam. Azad Univ. 2006, 16, 6–11. [Google Scholar]
- Mizyed, S.A.; Ashram, M.; Saymeh, R.; Marji, D. A Thermodynamic study of the charge transfer complexes of iodine with different tert-butylcalix[4]crowns. Z. Naturforsch. 2005, 60b, 1133–1137. [Google Scholar] [CrossRef]
- An, D.; Li, L.; Zhang, Z.; Asiri, A.M.; Alamry, K.A.; Zhang, X. Amino-bridged covalent organic polycalix[4]arenes for ultra efficient adsorption of iodine in water. Mat. Chem. Phys. 2020, 239, 122328. [Google Scholar] [CrossRef]
- Shetty, D.; Raya, J.; Han, D.S.; Asfari, Z.; Olsen, J.-C.; Trabolsi, A. Lithiated polycalix[4]arenes for efficient adsorption of iodine from solution and vapor phases. Chem. Mater. 2017, 29, 8968–8972. [Google Scholar] [CrossRef]
- Sharma, P.R.; Pandey, S.; Soni, V.K.; Choudhary, G.; Sharma, R.K. Macroscopic recognition of iodide by polymer appended calix[4]amidocrown resin. Supramol. Chem. 2019, 31, 634–644. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; An, D.; Li, H.; Zhang, X. Triazine-based covalent organic polycalix[4]arenes for highly efficient and reversible iodine capture in water. J. Mater. Sci. 2020, 55, 1854–1864. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Trabolsi, A. Pollutant removal with organic macrocycle-based covalent organic polymers and frameworks. Chem 2021, 7, 882–918. [Google Scholar] [CrossRef]
- Su, K.; Wang, W.; Li, B.; Yuan, D. Azo-Bridged Calix[4]resorcinarene-Based Porous Organic Frameworks with Highly Efficient Enrichment of Volatile Iodine. ACS Sustain. Chem. Eng. 2018, 6, 17402–17409. [Google Scholar] [CrossRef]
- Moulay, S. Molecular iodine/complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Koerner, J.C.; George, M.J.; Meyer, D.R.; Rosco, M.G.; Habib, M.M. Povidone-iodine concentration and dosing in cataract surgey. Surv. Ophthalmol. 2018, 63, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Siggia, S. The chemistry of polyvinylpyrrolidone-iodine. J. Am. Pharm. Assoc. 1957, 46, 201–204. [Google Scholar] [CrossRef]
- Bhagwat, D.; Oshlack, B. Stabilized PVP-I Solutions. U.S. Patent 5,126,127, 30 June 1992. [Google Scholar]
- Isenberg, S.J.; Apt, L.; Yoshimori, R.; Pham, C.; Lam, N.K. Efficacy of topical Povidone-Iodine during the first week after ophthalmic surgery. Am. J. Ophthalmol. 1997, 124, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, D.; Iny, O.; Pedi, F., Jr. Stabilizing Packaged Iodophor and Minimizing Leaching of Iodine through Packaging. EP Patent 0371283 A2, 6 June 1990. [Google Scholar]
- Zhu, W.; Gou, P.; Shen, Z. Applications of calixarenes in polymer synthesis. Macromol. Symp. 2008, 261, 74–84. [Google Scholar] [CrossRef]
- Zheng, Y.-S.; Ying, L.-Q.; Shen, Z.-Q. Polymerization of propylene oxide by a new neodymium complex of calixarene derivative. Polymer 2000, 41, 1641–11643. [Google Scholar] [CrossRef]
- Feng, W.; Yuan, L.H.; Zheng, S.Y.; Huang, G.L.; Qiao, J.L.; Zhou, Y. The effect of p-tert-butylcalix[n]arene on γ-radiation degradation of polypropylene. Radiat. Phys. Chem. 2000, 57, 425–429. [Google Scholar] [CrossRef]
- Mattiuzzi, A.; Troian-Gautier, L.; Mertens, J.; Reniers, F.; Bergamini, J.-F.; Lenne, Q.; Lagrost, C.; Jabin, I. Robust hydrophobic gold, glass and polypropylene surfaces obtained through a nanometric covalently bound organic layer. RSC Adv. 2020, 10, 13553–13561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennakesavulu, K.; Raviathul Basariya, M.; Sreedevi, P.; Raju, G.B.; Prabhakar, S.; Rao, S.S. Study on thermal decomposition of calix[4]arene and its application in thermal stability of polypropylene. Thermochim. Acta 2011, 515, 24–31. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Collins, E.M.; Deasy, M.; Ferguson, G.; Harris, S.J.; Kaitner, B.; Lough, A.J.; McKervey, M.A.; Marques, E.; Ruh, B.L.; et al. Synthesis, X-ray crystal structures, and cation-binding properties of alkyl calixaryl esters and ketones, a new family of macrocyclic molecular receptors. J. Am. Chem. Soc. 1989, 111, 8681–8691. [Google Scholar] [CrossRef]














| Wavelength (nm) | Absorbance Reduction % | ||
|---|---|---|---|
| 1:2 Molar Ratio | 1:6 Molar Ratio | 1:10 Molar Ratio | |
| 288 | −36.4% | −61.0% | −93.4% |
| 352 | −35.0% | −61.0% | −94.3% |
| 457 | −66.3% | −92.5 % | −97.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreri, L.; Rapisarda, M.; Leanza, M.; Munzone, C.; D’Antona, N.; Consoli, G.M.L.; Rizzarelli, P.; Spina, E.T.A. Calix[4]arene Derivative for Iodine Capture and Effect on Leaching of Iodine through Packaging. Molecules 2023, 28, 1869. https://doi.org/10.3390/molecules28041869
Ferreri L, Rapisarda M, Leanza M, Munzone C, D’Antona N, Consoli GML, Rizzarelli P, Spina ETA. Calix[4]arene Derivative for Iodine Capture and Effect on Leaching of Iodine through Packaging. Molecules. 2023; 28(4):1869. https://doi.org/10.3390/molecules28041869
Chicago/Turabian StyleFerreri, Loredana, Marco Rapisarda, Melania Leanza, Cristina Munzone, Nicola D’Antona, Grazia Maria Letizia Consoli, Paola Rizzarelli, and Emanuela Teresa Agata Spina. 2023. "Calix[4]arene Derivative for Iodine Capture and Effect on Leaching of Iodine through Packaging" Molecules 28, no. 4: 1869. https://doi.org/10.3390/molecules28041869