Dye-Encapsulated Metal–Organic Frameworks for the Multi-Parameter Detection of Temperature
Abstract
:1. Introduction
2. Results and Discussion
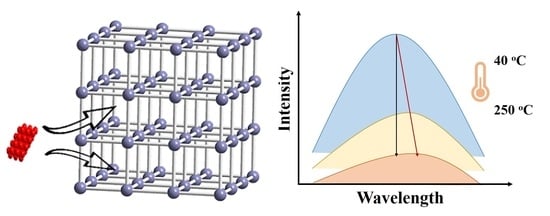
2.1. Structure of Zn-CYMPN
2.2. Thermostability
2.3. Quantum Yields
2.4. Fluorescent Properties
2.5. Fluorescent Temperature Detection
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, X.-D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980. [Google Scholar] [CrossRef]
- Jaque, D.; Vetrone, F. Luminescence nanothermometry. Nanoscale 2012, 4, 4301–4326. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chameleon Luminophore for Sensing Temperatures: Control of Metal-to-Metal and Energy Back Transfer in Lanthanide Coordination Polymers. Angew. Chem. Int. Ed. 2013, 52, 6413–6416. [Google Scholar] [CrossRef] [PubMed]
- Antić, Ž.; Dramićanin, M.D.; Prashanthi, K.; Jovanović, D.; Kuzman, S.; Thundat, T. Pulsed Laser Deposited Dysprosium-Doped Gadolinium–Vanadate Thin Films for Noncontact, Self-Referencing Luminescence Thermometry. Adv. Mater. 2016, 28, 7745–7752. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Kaczmarek, A.M.; Artizzu, F.; Abalymov, A.; Skirtach, A.G.; Van Hecke, K.; Van Deun, R. Luminescent PMMA Films and PMMA@SiO2 Nanoparticles with Embedded Ln(3+) Complexes for Highly Sensitive Optical Thermometers in the Physiological Temperature Range**. Chem. Eur. J. 2021, 27, 6479–6488. [Google Scholar] [CrossRef] [PubMed]
- Bednarkiewicz, A.; Marciniak, L.; Carlos, L.D.; Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 2020, 12, 14405–14421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, K.Y.; Tong, X.; Liu, Y.; Hu, C.; Liu, S.; Yu, Q.; Zhao, Q.; Huang, W. Phosphorescent Polymeric Thermometers for In Vitro and In Vivo Temperature Sensing with Minimized Background Interference. Adv. Funct. Mater. 2016, 26, 4386–4396. [Google Scholar] [CrossRef]
- Xia, T.; Shao, Z.; Yan, X.; Liu, M.; Yu, L.; Wan, Y.; Chang, D.; Zhang, J.; Zhao, D. Tailoring the triplet level of isomorphic Eu/Tb mixed MOFs for sensitive temperature sensing. Chem. Commun. 2021, 57, 3143–3146. [Google Scholar] [CrossRef]
- Marciniak, L.; Trejgis, K. Luminescence lifetime thermometry with Mn3+–Mn4+ co-doped nanocrystals. J. Mater. Chem. C 2018, 6, 7092–7100. [Google Scholar] [CrossRef]
- Cao, W.; Cui, Y.; Yang, Y.; Qian, G. Dyes Encapsulated Nanoscale Metal–Organic Frameworks for Multimode Temperature Sensing with High Spatial Resolution. ACS Mater. Lett. 2021, 3, 1426–1432. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Kumar Singh, A.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Koumura, T.N.M.a.N. Development of Next-Generation Organic-Based Solar Cells: Studies on Dye-Sensitized and Perovskite Solar Cells. Adv. Energy Mater. 2018, 9, 1802967. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Q.; Du, Y.; Zhou, X.; Du, X.; Wu, Q.; Lin, L.; Song, Y.; Li, F.; Yang, C.; et al. Metabolic Labeling of Peptidoglycan with NIR-II Dye Enables In Vivo Imaging of Gut Microbiota. Angew. Chem. Int. Ed. 2020, 59, 2628–2633. [Google Scholar] [CrossRef]
- Liang, T.; Wang, Q.; Li, Z.; Wang, P.; Wu, J.; Zuo, M.; Liu, Z. Removing the Obstacle of Dye-Sensitized Upconversion Luminescence in Aqueous Phase to Achieve High-Contrast Deep Imaging In Vivo. Adv. Funct. Mater. 2020, 30, 1910765. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, Y.; Wang, L.; Lv, Y.; Liu, J.; Zhou, T.-L.; Xie, R.-J. Color-Tunable and High-Efficiency Dye-Encapsulated Metal–Organic Framework Composites Used for Smart White-Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 18910–18917. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, H.; Wang, K.; Gao, Z.; Zhang, C.; Liu, X.; Zhao, Y.S.; Hu, F. Suppressing Nonradiative Processes of Organic Dye with Metal–Organic Framework Encapsulation toward Near-Infrared Solid-State Microlasers. ACS Appl. Mater. Interfaces 2018, 10, 35455–35461. [Google Scholar] [CrossRef]
- Yeroslavsky, G.; Umezawa, M.; Okubo, K.; Nigoghossian, K.; Thi Kim Dung, D.; Miyata, K.; Kamimura, M.; Soga, K. Stabilization of indocyanine green dye in polymeric micelles for NIR-II fluorescence imaging and cancer treatment. Biomater. Sci. 2020, 8, 2245–2254. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Cui, Y.; Xu, S.; Gong, J. Core–Shell Structured Cyclodextrin Metal–Organic Frameworks with Hierarchical Dye Encapsulation for Tunable Light Emission. Chem. Mater. 2019, 31, 1289–1295. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.; Yao, D.; Bian, K.; Zeng, W.; Liu, K.; Wang, D.; Zhang, B. An aggregation-induced emission dye-powered afterglow luminogen for tumor imaging. Chem. Sci. 2020, 11, 419–428. [Google Scholar] [CrossRef]
- McFarlane, T.D.; De Castro, C.S.; Holliman, P.J.; Davies, M.L. Improving the light harvesting and colour range of methyl ammonium lead tri-bromide (MAPbBr3) perovskite solar cells through co-sensitisation with organic dyes. Chem. Commun. 2019, 55, 35–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leea, W.; Kima, D.; Leea, S.; Parka, J.; Oha, S.; Kima, G.; Limb, J.; Kima, J. Stimuli-responsive switchable organic-inorganic nanocomposite materials. Nano Today 2018, 23, 97–123. [Google Scholar] [CrossRef]
- Liu, D.; Zou, D.; Zhu, H.; Zhang, J. Mesoporous Metal-Organic Frameworks: Synthetic Strategies and Emerging Applications. Small 2018, 14, 1801454. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, H.; Liu, Y.; Zhang, C.; Hu, F.; Zhao, Y.S. Dual-Wavelength Lasing from Organic Dye Encapsulated Metal-Organic Framework Microcrystals. Chem. Commun. 2019, 55, 3445–3448. [Google Scholar] [CrossRef]
- Xu, B.; Gao, Z.; Wei, Y.; Liu, Y.; Sun, X.; Zhang, W.; Wang, X.; Wang, Z.; Meng, X. Dynamically wavelength-tunable random lasers based on metal-organic framework particles. Nanoscale 2020, 12, 4833–4838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Sing, C.E.; Kunzelman, J.; Weder, C. Time–temperature indicators for high temperature applications. J. Mater. Chem. 2009, 19, 104–110. [Google Scholar] [CrossRef]
- Rocha, J.; Brites, C.D.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Qin, J.-H.; Qin, W.-J.; Xiao, Z.; Yang, J.-K.; Wang, H.-R.; Yang, X.-G.; Li, D.-S.; Ma, L.-F. Efficient Energy-Transfer-Induced High Photoelectric Conversion in a Dye-Encapsulated Ionic Pyrene-Based Metal–Organic Framework. Inorg. Chem. 2021, 60, 18593–18597. [Google Scholar] [CrossRef]
- Qin, J.-H.; Zhang, J.-R.; Xiao, Z.; Wu, Y.-P.; Xu, H.-M.; Yang, X.-G.; Ma, L.-F.; Li, D.-S. Topology- and Guest-Dependent Photoelectric Conversion of 2D Anionic Pyrene-Based Metal–Organic Framework. Cryst. Growth Des. 2022, 22, 4018–4024. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, L.; Han, L.; Zhang, Z.; Zhao, H. Synthesis, structure and characterization of two solvatochromic metal–organic frameworks for chemical-sensing applications. CrystEngComm 2018, 20, 2237–2240. [Google Scholar] [CrossRef]
- Lv, X.-L.; Xie, L.-H.; Wang, B.; Zhao, M.; Cui, Y.; Li, J.-R. Flexible metal–organic frameworks for the wavelength-based luminescence sensing of aqueous pH. J. Mater. Chem. C 2018, 6, 10628–10639. [Google Scholar] [CrossRef]
- Chen, L.; Ye, J.-W.; Wang, H.-P.; Pan, M.; Yin, S.-Y.; Wei, Z.-W.; Zhang, L.-Y.; Wu, K.; Fan, Y.-N.; Su, C.-Y. Ultrafast water sensing and thermal imaging by a metal-organic framework with switchable luminescence. Nat. Commun. 2017, 8, 15985. [Google Scholar] [CrossRef] [Green Version]
- Douvali, A.; Tsipis, A.C.; Eliseeva, S.V.; Petoud, S.; Papaefstathiou, G.S.; Malliakas, C.D.; Papadas, I.; Armatas, G.S.; Margiolaki, I.; Kanatzidis, M.G.; et al. Turn-on luminescence sensing and real-time detection of traces of water in organic solvents by a flexible metal-organic framework. Angew. Chem. Int. Ed. 2015, 54, 1651–1656. [Google Scholar] [CrossRef] [PubMed]




| Sample | Concentration of DPEE (mol/L) | Concentration of DPEM (mol/L) | Loading Density of DPEE | Loading Density of DPEM | Quantum Yield |
|---|---|---|---|---|---|
| Zn-CYMPN⊃DPEE-1 | 1 × 10−1 | / | 4.20 wt% | / | 17.87% |
| Zn-CYMPN⊃DPEE-2 | 1 × 10−2 | / | 0.63 wt% | / | 16.71% |
| Zn-CYMPN⊃DPEE-3 | 1 × 10−3 | / | 0.26 wt% | / | 3.20% |
| Zn-CYMPN⊃DPEM-1 | / | 1 × 10−1 | / | 3.40 wt% | 30.40% |
| Zn-CYMPN⊃DPEM-2 | / | 1 × 10−2 | / | 3.07 wt% | 5.45% |
| Zn-CYMPN⊃DPEM-3 | / | 1 × 10−3 | / | 2.91 wt% | 2.47% |
| DPEE | / | / | / | / | 1.19% |
| DPEM | / | / | / | / | 2.13% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Li, Y.; Yue, D. Dye-Encapsulated Metal–Organic Frameworks for the Multi-Parameter Detection of Temperature. Molecules 2023, 28, 729. https://doi.org/10.3390/molecules28020729
Wan Y, Li Y, Yue D. Dye-Encapsulated Metal–Organic Frameworks for the Multi-Parameter Detection of Temperature. Molecules. 2023; 28(2):729. https://doi.org/10.3390/molecules28020729
Chicago/Turabian StyleWan, Yating, Yanping Li, and Dan Yue. 2023. "Dye-Encapsulated Metal–Organic Frameworks for the Multi-Parameter Detection of Temperature" Molecules 28, no. 2: 729. https://doi.org/10.3390/molecules28020729