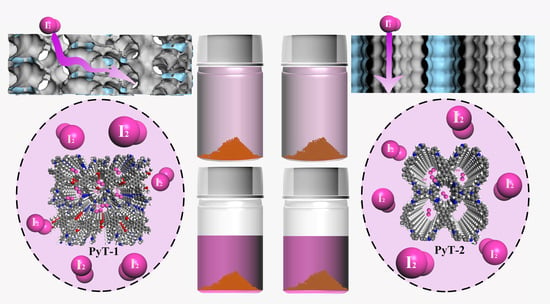
Polymorphic Covalent Organic Frameworks: Molecularly Defined Pore Structures and Iodine Adsorption Property
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PyT-1 and PyT-2
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dittmar, M. Nuclear energy: Status and future limitations. Energy 2012, 37, 35–40. [Google Scholar] [CrossRef]
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima nuclear accidents: A review of the environmental impacts. Sci. Total Environ. 2014, 470–471, 800–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Pan, T.; Xie, J.; Wu, F.; Dong, X.; Wang, S. Superior Iodine Uptake Capacity Enabled by an Open Metal-Sulfide Framework Composed of Three Types of Active Sites. CCS Chem. 2022, 1–9. [Google Scholar] [CrossRef]
- Yamashita, S.; Suzuki, S. Risk of thyroid cancer after the Fukushima nuclear power plant accident. Respir. Investig. 2013, 51, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L., Jr. Materials and processes for the effective capture and immobilization of radioiodine: A review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, X.; Zhou, Z.; Huang, T.; Wang, Y.; Xie, S.; Zeng, Z.; Tang, B.Z. Spiro-fused bicyclo[3,2,2] octatriene-cored triptycene: Synthesis, molecular packing, and functional aggregates. Sci. China Ser. B Chem. 2021, 64, 1976–1984. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Li, A. Biomass derived porous carbon for efficient capture of carbon dioxide, organic contaminants and volatile iodine with exceptionally high uptake. Chem. Eng. J. 2019, 372, 65–73. [Google Scholar] [CrossRef]
- Banerjee, D.; Chen, X.; Lobanov, S.S.; Plonka, A.M.; Chan, X.; Daly, J.A.; Kim, T.; Thallapally, P.K.; Parise, J.B. Iodine Adsorption in Metal Organic Frameworks in the Presence of Humidity. ACS Appl. Mater. Interfaces 2018, 10, 10622–10626. [Google Scholar] [CrossRef]
- Sun, H.; La, P.; Zhu, Z.; Liang, W.; Yang, B.; Li, A. Capture and reversible storage of volatile iodine by porous carbon with high capacity. J. Mater. Sci. 2015, 50, 7326–7332. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Q.; Li, Z.; Jiang, W.; Jiang, Q.; Jiang, D. Exceptional Iodine Capture in 2D Covalent Organic Frameworks. Adv. Mater. 2018, 30, e1801991. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, L.; Wang, K.; Wang, Z.; Liu, G.; Zhao, Y.; Zeng, Y. Two-dimensional covalent–organic frameworks for ultrahigh iodine capture. J. Mater. Chem. A 2020, 8, 9523–9527. [Google Scholar] [CrossRef]
- Lee, S.H.; Takahashi, Y. Selective immobilization of iodide onto a novel bismuth-impregnated layered mixed metal oxide: Batch and EXAFS studies. J. Hazard. Mater. 2020, 384, 121223. [Google Scholar] [CrossRef]
- Garino, T.J.; Nenoff, T.M.; Krumhansl, J.L.; Rademacher, D.X. Low-Temperature Sintering Bi-Si-Zn-Oxide Glasses for Use in Either Glass Composite Materials or Core/Shell I-129 Waste Forms. J. Am. Ceram. Soc. 2011, 94, 2412–2419. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Q.; Lin, Y.; Antonova, E.; Bensch, W.; Patzke, G.R. One-step hydrothermal synthesis of hierarchical Ag/Bi2WO6 composites: In situ growth monitoring and photocatalytic activity studies. Sci. China Chem. 2013, 56, 435–442. [Google Scholar] [CrossRef]
- Bruno, A.C.M. Evaluation of Silver Zeolites Sorbents Toward Their Ability to Promote Stable CH3I Storage as AgI Precipitates. ACS Appl. Mater. Interfaces 2017, 9, 25194–25203. [Google Scholar]
- Nenoff, T.M.; Rodriguez, M.A.; Soelberg, N.R.; Chapman, K.W. Silver-mordenite for radiologic gas capture from complex streams: Dual catalytic CH3I decomposition and I confinement. Microporous Mesoporous Mater. 2014, 200, 297–303. [Google Scholar] [CrossRef]
- Yan, Z.; Yuan, Y.; Tian, Y.; Zhang, D.; Zhu, G. Highly Efficient Enrichment of Volatile Iodine by Charged Porous Aromatic Frameworks with Three Sorption Sites. Angew. Chem. 2015, 54, 12733–12737. [Google Scholar] [CrossRef]
- Falaise, C.; Volkringer, C.; Facqueur, J.; Bousquet, T.; Gasnot, L.; Loiseau, T. Capture of iodine in highly stable metal–organic frameworks: A systematic study. Chem. Commun. 2013, 49, 10320–10322. [Google Scholar] [CrossRef]
- Liao, Y.; Weber, J.; Mills, B.M.; Ren, Z.; Faul, C.F.J. Highly Efficient and Reversible Iodine Capture in Hexaphenylbenzene-Based Conjugated Microporous Polymers. Macromolecules 2016, 49, 6322–6333. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, C.; Wang, H.; Jin, B.; Zhang, P.; Li, L.; Miao, S. Synthesis of N-containing porous aromatic frameworks via Scholl reaction for reversible iodine capture. Microporous Mesoporous Mater. 2021, 310, 110596. [Google Scholar] [CrossRef]
- Chen, R.; Hu, T.; Li, Y. Stable nitrogen-containing covalent organic framework as porous adsorbent for effective iodine capture from water. React. Funct. Polym. 2020, 159, 104806. [Google Scholar] [CrossRef]
- Dang, Q.-Q.; Wang, X.-M.; Zhan, Y.-F.; Zhang, X.-M. An azo-linked porous triptycene network as an absorbent for CO2 and iodine uptake. Polym. Chem. 2015, 7, 643–647. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, S.; Zhang, W.; Xu, B.; Tian, W. Covalent Organic Frameworks with Electron-Rich and Electron-Deficient Structures as Water Sensing Scaffolds. Macromol. Rapid Commun. 2020, 41, e2000003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Meng, L.; Ma, W.; Pan, G.; Zhang, W.; Zou, Y.; Liu, L.; Xu, B.; Tian, W. Dual-functional two-dimensional covalent organic frameworks for water sensing and harvesting. Mater. Chem. Front. 2021, 5, 4193–4201. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, L.; Ma, W.; Qi, Q.; Zhang, W.; Xu, B.; Liu, L.; Tian, W. Morphology controllable conjugated network polymers based on AIE-active building block for TNP detection. Chin. Chem. Lett. 2021, 32, 1037–1040. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, L.; Sun, Z.; Deng, H. Covalent organic frameworks for optical applications. Aggregate 2021, 2, e24. [Google Scholar] [CrossRef]
- Liang, R.-R.; Cui, F.-Z.; A, R.-H.; Qi, Q.-Y.; Zhao, X. A Study on Constitutional Isomerism in Covalent Organic Frameworks: Controllable Synthesis, Transformation, and Distinct Difference in Properties. CCS Chem. 2020, 2, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, C.; Jiang, S.; Ma, W.; Xu, B.; Liu, L.; Tian, W. A covalent organic polymer for turn-on fluorescence sensing of hydrazine. J. Mater. Chem. C 2021, 10, 2807–2813. [Google Scholar] [CrossRef]
- Gao, Q.; Li, X.; Ning, G.-H.; Xu, H.-S.; Liu, C.; Tian, B.; Tang, W.; Loh, K.P. Covalent Organic Framework with Frustrated Bonding Network for Enhanced Carbon Dioxide Storage. Chem. Mater. 2018, 30, 1762–1768. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Z.; Zhang, T.; Zhang, G.; Zhao, Z.; Wang, Y.; Chen, L. Direct pore engineering of 2D imine covalent organic frameworks <italic>via</italic> sub-stoichiometric synthesis. Sci. China Chem. 2022, 65, 190. [Google Scholar]
- Li, Y.; Guo, L.; Lv, Y.; Zhao, Z.; Ma, Y.; Chen, W.; Xing, G.; Jiang, D.; Chen, L. Polymorphism of 2D Imine Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2020, 60, 5363–5369. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, D.; Sun, F.; Zhuang, G.; Wang, Q.; Al-Enizi, A.M.; Ma, S. Substituent engineering in g-C3N4/COF heterojunctions for rapid charge separation and high photo-redox activity. Sci. China Chem. 2022, 65, 1704–1709. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, D. Covalent Organic Frameworks: New Materials Platform for Photocatalytic Degradation of Aqueous Pollutants. Materials 2021, 14, 5600. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, D.; Liu, J.; Liu, A.; Ma, D. Covalent Organic Frameworks: A Promising Materials Platform for Photocatalytic CO2 Reductions. Molecules 2020, 25, 2425. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhai, S.; Wang, Y.; Liu, A.; Chen, C. Synthetic Approaches for C-N Bonds by TiO2 Photocatalysis. Front. Chem. 2019, 7, 635. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2016, 87, 25. [Google Scholar] [CrossRef]
- Zhou, H.-G.; Xia, R.-Q.; Zheng, J.; Yuan, D.; Ning, G.-H.; Li, D. Acid-triggered interlayer sliding of two-dimensional copper(i)–organic frameworks: More metal sites for catalysis. Chem. Sci. 2021, 12, 6280–6286. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Z.; Wee, V.; Usadi, A.K.; Calabro, D.C.; Baugh, L.S.; Zhao, D. Interlayer Shifting in Two-Dimensional Covalent Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12995–13002. [Google Scholar] [CrossRef]
- Cai, S.; Sun, B.; Li, X.; Yan, Y.; Navarro, A.; Garzón-Ruiz, A.; Mao, H.; Chatterjee, R.; Yano, J.; Zhu, C.; et al. Reversible Interlayer Sliding and Conductivity Changes in Adaptive Tetrathiafulvalene-Based Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 19054–19061. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Xie, C.; Wang, H.; Liu, H.; Pan, Q.; Zhao, Y. Configurational Selectivity Study of Two-dimensional Covalent Organic Frameworks Isomers Containing D2h and C2 Building Blocks. Chem. Res. Chin. Univ. 2022, 38, 639–642. [Google Scholar] [CrossRef]
- Ji, C.; Su, K.; Wang, W.; Chang, J.; El-Sayed, E.-S.M.; Zhang, L.; Yuan, D. Tunable Cage-Based Three-Dimensional Covalent Organic Frameworks. CCS Chem. 2022, 4, 3095–3105. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.; Hao, W.; Li, Y.; Chen, L. Covalent Organic Frameworks Constructed from Flexible Building Blocks with High Adsorption Capacity for Pollutants. ACS Appl. Nano Mater. 2018, 1, 4756–4761. [Google Scholar] [CrossRef]
- Mokhtari, N.; Dinari, M. Developing novel amine-linked covalent organic frameworks towards reversible iodine capture. Sep. Purif. Technol. 2022, 301, 121948. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yuan, W.; Guo, X.; Bai, C.; Zou, Y.; Long, H.; Qi, Y.; Li, S.; Tao, G.; et al. Construction of Flexible Amine-linked Covalent Organic Frameworks by Catalysis and Reduction of Formic Acid via the Eschweiler–Clarke Reaction. Angew. Chem. Int. Ed. 2021, 60, 12396–12405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Zhang, H.; Liu, Y.; Cui, Y.; Jin, F.; Wang, K.; Liu, G.; Zhao, Y.; Zeng, Y. High iodine uptake in two-dimensional covalent organic frameworks. Chem. Commun. 2021, 57, 5558–5561. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Jiang, S.; Ma, W.; Liu, Z.; Liu, L.; Zou, Y.; Xu, B.; Tian, W. Polymorphic Covalent Organic Frameworks: Molecularly Defined Pore Structures and Iodine Adsorption Property. Molecules 2023, 28, 449. https://doi.org/10.3390/molecules28010449
Wang C, Jiang S, Ma W, Liu Z, Liu L, Zou Y, Xu B, Tian W. Polymorphic Covalent Organic Frameworks: Molecularly Defined Pore Structures and Iodine Adsorption Property. Molecules. 2023; 28(1):449. https://doi.org/10.3390/molecules28010449
Chicago/Turabian StyleWang, Canran, Shan Jiang, Wenyue Ma, Zhaoyang Liu, Leijing Liu, Yongcun Zou, Bin Xu, and Wenjing Tian. 2023. "Polymorphic Covalent Organic Frameworks: Molecularly Defined Pore Structures and Iodine Adsorption Property" Molecules 28, no. 1: 449. https://doi.org/10.3390/molecules28010449