Inhibition of Advanced Glycation End-Products by Tamarindus indica and Mitragyna inermis Extracts and Effects on Human Hepatocyte and Fibroblast Viability
Abstract
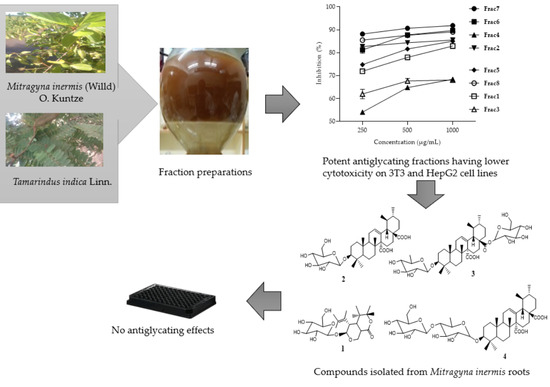
:1. Introduction
2. Results and Discussion
2.1. Antiglycation Activity of Mitragyna inermis and Tamarindus indica Fractions
2.2. Cytotoxicity Profile of Mitragyna inermis and Tamarindus indica Fractions in HepG2 and NIH-3T3 Cells
2.3. Compounds Isolated from M. inermis Fraction and Their Anti-Glycation Activities
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction and Isolation
3.3. Spectroscopic Data of the Isolated Compounds
3.4. Anti-Glycation Activity Assay
3.5. Cytotoxicity Assay
3.5.1. Cell Culture Procedure
3.5.2. Cytotoxicity Procedure
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, S.N.; Farzana, S.; Aleem, U.; Sheikh, S.; Tamfu, A.N.; Ashraf, S.; Ul-Haq, Z.; Ullah, S.; Wahab, A.; Choudhary, M.I.; et al. Peptide conjugates of 18β-glycyrrhetinic acid as potent inhibitors of α-glucosidase and AGEs-induced oxidation. Eur. J. Pharm. Sci. 2022, 168, 106045. [Google Scholar] [CrossRef] [PubMed]
- Younus, H.; Anwar, S. Prevention of Non-Enzymatic Glycosylation (Glycation): Implication in the Treatment of Diabetic Complication. Int. J. Health Sci. 2016, 10, 247–263. [Google Scholar] [CrossRef]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of Fructose in Glycation and Cross-Linking of Proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Schmidt, A.M. Protein glycation: A firm link to endothelial cell dysfunction. Circ. Res. 2004, 95, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Goh, S.Y.; Cooper, M.E. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, H.; Choudhary, M.I. Glycation, carbonyl stress and AGEs inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1267–1284. [Google Scholar] [CrossRef]
- Arbonnier, M. Arbres, Arbustes et Lianes Des Zones Sèches D’Afrique de L’Ouest; Cirad: Montpelier, France, 2002; pp. 2–87. [Google Scholar]
- Bonde, L. Densité Des Peupelements, Equations Allométriques, Gradient Climatique, Mode D’Utilisation Des Terres, Pfnl, Potentiel ÉConomique; Université Jozeph KI-ZERBO: Ouagadougou, Burkina Faso, 2019; pp. 1–180. [Google Scholar]
- Nacoulma, O.G. Plantes Médicinales et Pratiques Médicinales Traditionnelles: Cas Du Plateau Central; These d’Etat Univ. Ouagadougou: Ouagadougou, Burkina Faso, 1996; pp. 1–328. [Google Scholar]
- Zizka, A.; Thiombiano, A.; Dressler, S.; Nacoulma, B.I.; Ouédraogo, A.; Ouédraogo, I.; Ouédraogo, O.; Zizka, G.; Hahn, K.; Schmidt, M. Traditional plant use in Burkina Faso (West Africa): A national-scale analysis with focus on traditional medicine. J. Ethnobiol. Ethnomed. 2015, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Kinda, P.; Zerbo, P.; Guenné, S.; Compaoré, M.; Ciobica, A.; Kiendrebeogo, M. Medicinal Plants Used for Neuropsychiatric Disorders Treatment in the Hauts Bassins Region of Burkina Faso. Medicines 2017, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Adoum, O.A.; Nenge, H.P.; Chedi, B. the Steroidal Component and Hypoglycaemic Effect of Stem Bark of Mitragyna inermis (wild) O. Kundze (rubiaceae) in alloxan induced diabetic wistar rats. Int. J. Appl. Biol. Pharm. Technol. 2012, 3, 169–174. [Google Scholar]
- Funke, I.; Melzig, M.F. Traditionally used plants in diabetes therapy: Phytotherapeutics as inhibitors of alpha-amylase activity. Rev. Bras. Farmacogn. 2006, 16, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ouédraogo, R.J.; Somda, M.B.; Ouattara, L.; Kagambega, W.; Ouoba, P.; Ouédraogo, G.A. Evaluation of the antioxidant and A-amylase inhibitory activities of Mitragyna inermis (Willd) O. Kuntze and Tamarindus indica Linn. J. Exp. Biol. Agric. Sci. 2020, 8, 676–682. [Google Scholar] [CrossRef]
- Traore, F.; Gasquet, M.; Laget, M.; Guiraud, H.; Di Giorgio, C.; Azas, N.; Doumbo, O.; Timon-David, P. Toxicity and genotoxicity of antimalarial alkaloid rich extracts derived from Mitragyna inermis O. Kuntze and Nauclea latifolia. Phyther. Res. 2000, 14, 608–611. [Google Scholar] [CrossRef]
- Pimple, B.P.; Kadam, P.V.; Badgujar, N.S.; Bafna, A.R.; Pati, M.J. Protective effect of Tamarindus indica linn against paracetamol-induced hepatotoxicity in rats. Indian J. Pharm. Sci. 2007, 69, 827–831. [Google Scholar] [CrossRef] [Green Version]
- Ouedraogo, Y.; Guissou, I.P.; Nacoulma, O.G. Biological and Toxicological Study of Aqueous Root Extract from Mitragyna inermis (Willd oktze) Rubiaceae. Int. J. Pharmacol. 2007, 3, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Konkon, N.G.; Adjoungoua, A.L.; Manda, P.; Simaga, D.; N’Guessan, K.E.; Kone, B.D. Toxicological and phytochemical screening study of Mitragyna inermis (willd.) O ktze (Rubiaceae), anti diabetic plant. J. Med. Plants Res. 2008, 2, 279–284. [Google Scholar]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Muthenna, P.; Akileshwari, C.; Saraswat, M.; Bhanuprakash, G.R. Inhibition of advanced glycation end-product formation on eye lens protein by rutin. Br. J. Nutr. 2012, 107, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Park, J.J.; Min, B.S.; Jung, H.J.; Islam, M.N.; Choi, J.S. Inhibition of advanced glycation endproducts formation by Korean thistle, Cirsium maackii. Asian Pac. J. Trop. Med. 2015, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Farah, N.; Bukhari, S.A.; Ali, M.; Naqvi, S.A.R.; Mahmood, S. Phenolic acid profiling and antiglycation studies of leaf and fruit extracts of tyrosine primed Momordica charantia seeds for possible treatment of diabetes mellitus. Pak. J. Pharm. Sci. 2018, 31, 2667–2672. [Google Scholar]
- Safari, M.R.; Azizi, O.; Heidary, S.S.; Kheiripour, N.; Ravan, A.P. Antiglycation and antioxidant activity of four Iranian medical plant extracts. J. Pharmacopunct. 2018, 21, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, R.J.; Ouattara, L.; Kabre, P.; Sanou, Y.; Somda, M.B.; Ouoba, P.; Ouédraogo, G.A. Season and Ecotype Effects on Soluble Phenolic Compounds Content and Antioxidant Potential of Tamarindus indica and Mitragyna inermis. J. Pharm. Pharmacol. 2022, 10, 143–156. [Google Scholar] [CrossRef]
- Bhatia, V.K.; Gupta, S.R.; Seshadri, T.R. C-glycosides of tamarind leaves. Phytochemistry 1966, 5, 177–181. [Google Scholar] [CrossRef]
- Asase, A.; Kokubun, T.; Renée, J.G.; Kite, G.; Simmonds, M.S.J.; Oteng-Yeboah, A.A.; Odamtten, G.T. Chemical constituents and antimicrobial activity of medicinal plants from Ghana: Cassia sieberiana, Haematostaphis barteri, Mitragyna inermis and Pseudocedrela kotschyi. Phyther. Res. 2008, 22, 1013–1016. [Google Scholar] [CrossRef]
- Escalona-Arranz, J.C.; Pérez-Rosés, R.; Jiménez, I.L.; Rodríguez-Amado, J.; Argota-Coello, H.; Cañizares-Lay, J.; Morris-Quevedo, H.J.; Sierra-González, G. Chemical Constituents of Tamarindus indica L. leaves. Rev. Cuba. Química. 2010, 3, 65–71. [Google Scholar]
- Choudhary, R.K.; Swarnkar, P.L. Antioxidant activity of phenolic and flavonoid compounds in some medicinal plants of India. Nat. Prod. Res. 2011, 25, 1101–1109. [Google Scholar] [CrossRef]
- Jahan, H.; Siddiqui, N.N.; Iqbal, S.; Basha, F.Z.; Shaikh, S.; Pizzi, M.; Choudhary, M.I. Suppression of COX-2/PGE2 levels by carbazole-linked triazoles via modulating methylglyoxal-AGEs and glucose-AGEs—induced ROS/NF-κB signaling in monocytes. Cell. Signal. 2022, 97, 110372. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Ngene, A.A.; Anaga, A.O.; Chigor, V.N.; Henrietta, I.I.; Okoh, A.I. Acute toxicity and hepatotoxicokinetic studies of Tamarindus indica extract. Molecules 2011, 16, 7415–7427. [Google Scholar] [CrossRef] [Green Version]
- Timothy, S.; Wazis, C.; Helga, B.; Maina, A.; Bomai, H.I. Anticonvulsant Screening of the Aqueous and Ethanol Extracts OF Mitragyna inermis Bark. Int. J. Pharm. Ther. 2014, 5, 358–363. [Google Scholar]
- Beek, V.T.; Lankhorst, P.; Verpoorte, R.; Svendsen, A. Isolation of the Secoiridoid-Glucoside Sweroside from Tabernaemontana psorocarpa. Planta Med. 1982, 44, 30–31. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wei, W.; Nagao, S.; Mahmud, T.; Hori, K.; Kobayashi, M.; Uji, T.; Shibuya, H. Indonesian medicinal plants. XIV. Characterization of 3′-O- caffeoylsweroside, a new secoiridoid glucoside, and kelampayosides A and B, two new phenolic apioglucosides, from the bark of Anthocephalus chinensis (Rubiaceae). Chem. Pharm. Bull. 1996, 44, 1162–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquino, R.; De Simone, F.; Pizza, C.; Cerri, R.; De Mello, J.F. Quinovic acid glycosides from Guettarda platypoda. Phytochemistry 1988, 27, 2927–2930. [Google Scholar] [CrossRef]
- Kang, W.Y.; Wang, J.S.; Yang, X.S.; Hao, X.J. Triterpenoid saponins from Luculia pincia Hook. Chin. J. Chem. 2003, 21, 1501–1505. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yu, B.Y.; Yang, X.W.; Zhang, J. Triterpenoid saponins from bark Mitragyna inermis. Zhongguo Zhongyao Zazhi 2002, 27, 276–277. [Google Scholar]
- Kang, W.Y.; Du, Z.Z.; Hao, X.J. Triterpenoid saponins from Neonauclea sessilifolia Merr. J. Asian Nat. Prod. Res. 2004, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hao, X. Triterpenoid saponins from Mitragyna rotundifolia. Biochem. Syst. Ecol. 2006, 34, 585–587. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Yu, B.Y.; Yang, X.W. 27-Nor-triterpenoid glycosides from Mitragyna inermis. Phytochemistry 2002, 61, 379–382. [Google Scholar] [CrossRef]
- Checkouri, E.; Reignier, F.; Da Silva, C.R.; Meilhac, O. Evaluation of polyphenol content and antioxidant capacity of aqueous extracts from eight medicinal plants from reunion island: Protection against oxidative stress in red blood cells and preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I.; Shah, Z.; Khan, K.M.; Ur-Rahman, A. Derivatives of 6-Nitrobenzimidazole Inhibit Fructose-Mediated Protein Glycation and Intracellular Reactive Oxygen Species Production. Med. Chem. 2017, 13, 577–584. [Google Scholar] [CrossRef]




| Fraction (M. inermis or T. indica) | Concentration (µg/mL) | % Inhibition ±SD |
|---|---|---|
| Frac1 | 1000 | 82.87 ± 0.63 * |
| 500 | 77.94 ± 0.66 * | |
| 250 | 71.86 ± 1.01 * | |
| Frac2 | 1000 | 85.50 ± 0.17 *** |
| 500 | 84.30 ± 0.20 *** | |
| 250 | 82.63 ± 0.10 *** | |
| Frac3 | 1000 | 68.04 ± 0.78 ns |
| 500 | 67.64 ± 1.09 ns | |
| 250 | 61.95 ± 2.07 ns | |
| Frac4 | 1000 | 68.34 ± 0.47 |
| 500 | 64.74 ± 0.29 | |
| 250 | 53.99 ± 0.58 | |
| Frac5 | 1000 | 84.97 ± 0.26 ** |
| 500 | 81.59 ± 0.75 ** | |
| 250 | 74.77 ± 0.80 ** | |
| Frac6 | 1000 | 89.66 ± 0.23 *** |
| 500 | 87.72 ± 0.55 *** | |
| 250 | 81.19 ± 1.49 *** | |
| Frac7 | 1000 | 91.82 ± 0.21 **** |
| 500 | 90.60 ± 0.28 **** | |
| 250 | 88.12 ± 0.23 **** | |
| Frac8 | 1000 | 89.03 ± 0.25 *** |
| 500 | 87.56 ± 0.001 *** | |
| 250 | 85.45 ± 0.46 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouédraogo, R.J.; Aleem, U.; Ouattara, L.; Nadeem-ul-Haque, M.; Ouédraogo, G.A.; Jahan, H.; Shaheen, F. Inhibition of Advanced Glycation End-Products by Tamarindus indica and Mitragyna inermis Extracts and Effects on Human Hepatocyte and Fibroblast Viability. Molecules 2023, 28, 393. https://doi.org/10.3390/molecules28010393
Ouédraogo RJ, Aleem U, Ouattara L, Nadeem-ul-Haque M, Ouédraogo GA, Jahan H, Shaheen F. Inhibition of Advanced Glycation End-Products by Tamarindus indica and Mitragyna inermis Extracts and Effects on Human Hepatocyte and Fibroblast Viability. Molecules. 2023; 28(1):393. https://doi.org/10.3390/molecules28010393
Chicago/Turabian StyleOuédraogo, Relwendé Justin, Umair Aleem, Lassina Ouattara, Muhammad Nadeem-ul-Haque, Georges Anicet Ouédraogo, Humera Jahan, and Farzana Shaheen. 2023. "Inhibition of Advanced Glycation End-Products by Tamarindus indica and Mitragyna inermis Extracts and Effects on Human Hepatocyte and Fibroblast Viability" Molecules 28, no. 1: 393. https://doi.org/10.3390/molecules28010393