Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Photocatalysts by One-Pot Impregnation and Their Structural Characterization
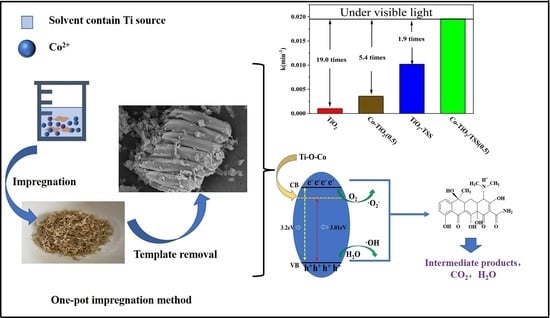
2.2. Analysis of Photocatalytic Activity
2.3. Cyclic Stability Test
2.4. Identification of the Active Species and Elucidation of Mechanism
2.5. Photocatalytic Reaction Mechanism
2.5.1. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.5.2. Ultraviolet–Visible (UV–vis) Diffuse Reflectance Spectral Analysis
2.5.3. Photoluminescence (PL) Spectroscopy
2.5.4. Electrochemical Analysis
2.5.5. Elucidation of Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalyst
3.3. Characterization of the Prepared Photocatalysts
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, A.K.; Hollmann, D.; Schwarze, M.; Panda, C.; Singh, B.; Menezes, P.W.; Indra, A. Exploring the Mechanism of Peroxodisulfate Activation with Silver Metavanadate to Generate Abundant Reactive Oxygen Species. Adv. Sustain. Syst. 2021, 5, 2000288. [Google Scholar] [CrossRef]
- Lin, Z.; Zhen, Z.; Luo, S.W.; Ren, L.; Chen, Y.J.; Wu, W.J.; Zhang, W.J.; Liang, Y.Q.; Song, Z.G.; Li, Y.T.; et al. Effects of two ecological earthworm species on tetracycline degradation performance, pathway and bacterial community structure in laterite soil. J. Hazard. Mater. 2021, 412, 125212. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, J.; Li, Y.B.; Ding, Z.L.; Huang, J.G. A biotemplate synthesized hierarchical Sn-doped TiO2 with superior photocatalytic capacity under simulated solar light. Ceram. Int. 2021, 47, 8218–8227. [Google Scholar] [CrossRef]
- Amri, F.; Septiani, N.L.W.; Rezki, M.; Iqbal, M.; Yamauchi, Y.; Golberg, D.; Kaneti, Y.V.; Yuliarto, B. Mesoporous TiO2-based architectures as promising sensing materials towards next-generation biosensing applications. J. Mater. Chem. B 2021, 9, 1189–1207. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Han, Y.; Yang, C.; Ma, Y.; Du, C.; Teng, Q.; Liu, H.; Zhong, Y. Spatial separation of photogenerated carriers and enhanced photocatalytic performance on Ag3PO4 catalysts via coupling with PPy and MWCNTs. Appl. Catal. B Environ. 2019, 258, 117969. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, C.; Wu, S.; Li, X.; Chen, Y.; Yang, W.L. Construction of Built-In Electric Field within Silver Phosphate Photocatalyst for Enhanced Removal of Recalcitrant Organic Pollutants. Adv. Funct. Mater. 2020, 30, 2002918. [Google Scholar] [CrossRef]
- Mu, J.B.; Chen, B.; Zhang, M.Y.; Guo, Z.C.; Zhang, P.; Zhang, Z.Y.; Sun, Y.Y.; Shao, C.L.; Liu, Y.C. Enhancement of the Visible-Light Photocatalytic Activity of In2O3-TiO2 Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2012, 4, 424–430. [Google Scholar] [CrossRef]
- Eshaghi, A.; Eshaghi, A. Investigation of superhydrophilic mechanism of titania nano layer thin film-Silica and indium oxide dopant effect. Bull. Mater. Sci. 2012, 35, 137–142. [Google Scholar] [CrossRef]
- Liu, L.C.; Ji, Z.Y.; Zou, W.X.; Gu, X.R.; Deng, Y.; Gao, F.; Tang, C.J.; Dong, L. In Situ Loading Transition Metal Oxide Clusters on TiO2 Nanosheets As Co-catalysts for Exceptional High Photoactivity. Acs Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- Jostar, S.T.; Devadason, S.; Arputhavalli, G.J.; Jebasingh, S.; Suthagar, J. Enhancement of opto-electrical properties in Co doped CdS–TiO2 nanocomposite thin film as photoanode for Semiconductor Sensitized Solar Cells (SSSCs). Phys. E Low-Dimens. Syst. Nanostructures 2022, 142, 115287. [Google Scholar] [CrossRef]
- Binas, V.; Stefanopoulos, V.; Kiriakidis, G.; Papagiannakopoulos, P. Photocatalytic oxidation of gaseous benzene, toluene and xylene under UV and visible irradiation over Mn-doped TiO2 nanoparticles. J. Mater. 2019, 5, 56–65. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Li, J. Photocatalytic and microwave absorbing properties of polypyrrole/Fe-doped TiO2 composite by in situ polymerization method. J. Alloy. Compd. 2011, 509, 1953–1957. [Google Scholar] [CrossRef]
- Temam, E.G.; Djani, F.; Rahmane, S.; Ben Temam, H.; Gasmi, B. Photocatalytic activity of Al/Ni doped TiO2 films synthesized by sol-gel method: Dependence on thickness and crystal growth of photocatalysts. Surf. Interfaces 2022, 31, 102077. [Google Scholar] [CrossRef]
- Veerathangam, K.; Pandian, M.S.; Ramasamy, P. Incorporation of CO2+ in CdS quantum dots for solar cell applications. Mater. Sci. Semicond. Process. 2020, 108, 104869. [Google Scholar] [CrossRef]
- Sivagamai, D.; Geetha Priyadarshini, B. Composition dependent structural, morphological, optical and electrical properties of CdS:Co window layer grown by chemical bath deposition. Mater. Sci. Energy Technol. 2020, 3, 709–718. [Google Scholar] [CrossRef]
- Hamadanian, M.; Reisi-Vanani, A.; Majedi, A. Sol-Gel Preparation and Characterization of CO/TiO2 Nanoparticles: Application to the Degradation of Methyl Orange. J. Iran. Chem. Soc. 2010, 7, S52–S58. [Google Scholar] [CrossRef]
- El Mragui, A.; Zegaoui, O.; Esteves da Silva, J.C.G. Elucidation of the photocatalytic degradation mechanism of an azo dye under visible light in the presence of cobalt doped TiO2 nanomaterials. Chemosphere 2021, 266, 128931. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Paineau, E.; Laachachi, A.; Colbeau-Justin, C.; Remita, H.; Ghazzal, M.N. A sol-gel biotemplating route with cellulose nanocrystals to design a photocatalyst for improving hydrogen generation. J. Mater. Chem. A 2020, 8, 10779–10786. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.B.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Li, C.Y.; Paineau, E.; Habibi, Y.; Remita, H.; Colbeau-Justin, C.; Ghazzal, M.N. Enhanced Photogenerated Charge Carriers and Photocatalytic Activity of Biotemplated Mesoporous TiO2 Films with a Chiral Nematic Structure. Chem. Mater. 2019, 31, 4851–4863. [Google Scholar] [CrossRef]
- Gao, L.K.; Gan, W.T.; Qiu, Z.; Cao, G.L.; Zhan, X.X.; Qiang, T.G.; Li, J. Biomorphic Carbon-Doped TiO2 for Photocatalytic Gas Sensing with Continuous Detection of Persistent Volatile Organic Compounds. Acs Appl. Nano Mater. 2018, 1, 1766–1775. [Google Scholar] [CrossRef]
- Xu, Y.G.; Liu, J.; Xie, M.; Jing, L.Q.; Xu, H.; She, X.J.; Li, H.M.; Xie, J.M. Construction of novel CNT/LaVO4 nanostructures for efficient antibiotic photodegradation. Chem. Eng. J. 2019, 357, 487–497. [Google Scholar] [CrossRef]
- Wei, Y.H.; Chen, H.; Jiang, H.J.; Wang, B.Y.; Liu, H.; Zhang, Y.; Wu, H. Biotemplate-Based Engineering of High-Temperature Stable Anatase TiO2 Nanofiber Bundles with Impregnated CeO2 Nanocrystals for Enhanced Lithium Storage. Acs Sustain. Chem. Eng. 2019, 7, 7823–7832. [Google Scholar] [CrossRef]
- Yang, H.Y.; Jiang, L.; Li, Y.Z.; Li, G.Q.; Yang, Y.P.; He, J.; Wang, J.Q.; Yan, Z.Y. Highly Efficient Red Cabbage Anthocyanin Inserted TiO2 Aerogel Nanocomposites for Photocatalytic Reduction of Cr(VI) under Visible Light. Nanomaterials 2018, 811, 937. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, Y.H.; You, Y.; Zeng, C.; Xiao, X.; Ma, T.Y.; Huang, H.W. Bifunctional Hydrogen Production and Storage on 0D-1D Heterojunction of Cd0.5Zn0.5S@Halloysites. Adv. Funct. Mater. 2019, 29, 1903825. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martinez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Jiang, L.; He, J.; Yang, Y.; Mao, D.; Chen, D.; Wang, W.; Chen, Y.; Sharma, V.K.; Wang, J. Enhancing visible-light photocatalytic activity of hard-biotemplated TiO2: From macrostructural morphology replication to microstructural building units design. J. Alloy. Compd. 2022, 898, 162886. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Li, G.; Xue, C.; Guo, W. Hetero-structural NiTiO3/TiO2 nanotubes for efficient photocatalytic hydrogen generation. Renew. Energy 2017, 111, 410–415. [Google Scholar] [CrossRef]
- Wu, B.; Shan, C.; Zhang, X.; Zhao, H.; Ma, S.; Shi, Y.; Yang, J.; Bai, H.; Liu, Q. CeO2/Co3O4 porous nanosheet prepared using rose petal as biotemplate for photo-catalytic degradation of organic contaminants. Appl. Surf. Sci. 2021, 543, 148677. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Li, Y.; Wang, W.; Li, J.; Li, J.; Ao, Y.; He, J.; Sharma, V.K.; Wang, J. Morphology- and Phase-Controlled Synthesis of Visible-Light-Activated S- doped TiO2 with Tunable S4+/S6+ Ratio. Chem. Eng. J. 2020, 402, 125549. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Wang, B.; An, H.; Chen, Z.; Cui, H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O–TiO2 composite. Appl. Clay Sci. 2016, 119, 311–320. [Google Scholar] [CrossRef]
- Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Jia, J.; Tian, J.; Li, Z.; Wang, X.; Xing, Z. Oxygen vacancy-mediated sandwich-structural TiO(2-x) /ultrathin g-C(3)N(4)/TiO(2-x) direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Hazard Mater 2021, 408, 124432. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, J.; Ma, H.; Zang, L.; Li, D.; Guo, S.; Ci, Y. Preparation of heterogeneous TiO2/g-C3N4 with a layered mosaic stack structure by use of montmorillonite as a hard template approach: TC degradation, kinetic, mechanism, pathway and DFT investigation. Appl. Clay Sci. 2021, 207, 106107. [Google Scholar] [CrossRef]
- Calliari, L.; Fanchenko, S.; Filippi, M. Effective medium theory for REELS analysis of amorphous carbon films. Surf. Interface Anal. 2010, 42, 1066–1071. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Luo, B.; Sun, D.; Yan, X.; Xue, Q. Surface amorphization and deoxygenation of graphene oxide paper by Ti ion implantation. Carbon 2011, 49, 3141–3147. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Su, T.; Ji, H.; Qin, Z. Co3O4/CdS p-n heterojunction for enhancing photocatalytic hydrogen production: Co-S bond as a bridge for electron transfer. Appl. Surf. Sci. 2021, 567, 150849. [Google Scholar] [CrossRef]
- Ma, X.; Miao, L.; Bie, S.; Jiang, J. Synergistic effect of V/N-codoped anatase TiO2 photocatalysts. Solid State Commun. 2010, 150, 689–692. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Li, B.; Yan, L.; Xing, Z.; Li, J. Enhanced visible light activity and mechanism of TiO2 codoped with molybdenum and nitrogen. Mater. Sci. Eng. B 2013, 178, 425–430. [Google Scholar] [CrossRef]
- Naseem, S.; Pinchuk, I.V.; Luo, Y.K.; Kawakami, R.K.; Khan, S.; Husain, S.; Khan, W. Epitaxial growth of cobalt doped TiO2 thin films on LaAlO3(100) substrate by molecular beam epitaxy and their opto-magnetic based applications. Appl. Surf. Sci. 2019, 493, 691–702. [Google Scholar] [CrossRef]
- Zhu, J.F.; Chen, F.; Zhang, J.L.; Chen, H.J.; Anpo, M. Fe3+-TiO2 photocatalysts prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A-Chem. 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Poirot, N.; Brize, V. Room-temperature ferromagnetism observed in undoped semiconducting and insulating oxide thin films. Phys. Rev. B 2006, 73, 132404. [Google Scholar] [CrossRef]
- Han, H.; Frei, H. Visible light absorption of binuclear TiOCoII charge-transfer unit assembled in mesoporous silica. Microporous Mesoporous Mater. 2007, 103, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wang, C.; Lei, J. Photocatalytic properties of anatase silver-doped titanium dioxide in UV and simulated solar light. Mater. Eng. 2020, 48, 579–583. [Google Scholar]
- Mohamed, H.H.; Al Qarni, F.; Alomair, N.A.; Akhtar, S. Solar Photocatalytic and Antimicrobial Activity of Porous Indium-Doped TiO2 Nanostructure. Arab. J. Sci. Eng. 2021, 46, 5505–5522. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Mahadik, M.A.; Chung, H.-S.; Chae, W.-S.; Cho, M.; Jang, J.S. CdIn2S4 chalcogenide/TiO2 nanorod heterostructured photoanode: An advanced material for photoelectrochemical applications. Appl. Surf. Sci. 2019, 490, 18–29. [Google Scholar] [CrossRef]
- He, X.; Kai, T.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]











| Sample | Light Source and Irradiation Time | Initial Concentration and Catalyst Dosage | Degradation Rate (%) | Ref. |
|---|---|---|---|---|
| Ag3PO4@MWCNTs@PPy | 300 W Xeon lamp, 2 min | 20 mg/L 0.5 g/L | 94.48 | [5] |
| Cu2O-TiO2-Pal | 500 W Xeon lamp, 4 h | 30 mg/L 1.0 g/L | 81.85 | [32] |
| TiO2-X/ultrathin g-C3N4/TiO2-X | 300 W Xeon lamp, 60 min | 10 mg/L 1.0 g/L | 87.70 | [33] |
| Co-TiO2/TSS | 5 W LED lamp, 90 min | 13 mg/L 0.5 h/L | 86.00 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Jiang, L.; Li, Y.; Wang, X.; Zhao, L.; Huang, P.; Chen, D.; Wang, J. Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk. Molecules 2023, 28, 386. https://doi.org/10.3390/molecules28010386
Li Q, Jiang L, Li Y, Wang X, Zhao L, Huang P, Chen D, Wang J. Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk. Molecules. 2023; 28(1):386. https://doi.org/10.3390/molecules28010386
Chicago/Turabian StyleLi, Quanhui, Liang Jiang, Yuan Li, Xiangrong Wang, Lixia Zhao, Pizhen Huang, Daomei Chen, and Jiaqiang Wang. 2023. "Enhancement of Visible-Light Photocatalytic Degradation of Tetracycline by Co-Doped TiO2 Templated by Waste Tobacco Stem Silk" Molecules 28, no. 1: 386. https://doi.org/10.3390/molecules28010386