An Assessment of Mass Flows, Removal and Environmental Emissions of Bisphenols in a Sequencing Batch Reactor Wastewater Treatment Plant
Abstract
:1. Introduction
| Treatment | Matrix/Removal | Year | Unit | BPA | BPAF | BPE | BPF | BPS | BPB | BPZ | BPAP | BPP | BPBP | BPC | BPG | BPPH | BPTMC | Total | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary & secondary treatment, disinfection | Influent | 2016 | ng·L−1 | 1920.71 | 1.50 | 7.13 | 50.57 | 85.64 | / | / | / | / | / | / | / | / | / | 2065.55 | [8] |
| Effluent | ng·L−1 | 223.71 | 1.45 | 8.70 | 6.69 | 1.34 | / | / | / | / | / | / | / | / | / | 241.90 | |||
| SS | ng·g−1 | 445.14 | 7.14 | 7.99 | 70.40 | 3.40 | / | / | / | / | / | / | / | / | / | 534.07 | |||
| Removal | % | 78.3 | −153 | −82.5 | 93.8 | 98.9 | / | / | / | / | / | / | / | / | / | 88.3 | |||
| Primary & secondary treatment | Influent | 2012 | ng·L−1 | 60.5 | 1.1 | / | 10.4 | 14.7 | 2.5 | 0.6 | 0.3 | 7.8 | / | / | / | / | / | 98.0 | [16] |
| Effluent | ng·L−1 | 5.2 | <LOD | / | 0.6 | 2.4 | 0.6 | <LOD | <LOD | 0.8 | / | / | / | / | / | 9.6 | |||
| PS & SS | ng·g−1 | 5.6 | <LOD | / | 8.2 | 185.7 | <LOD | <LOD | <LOD | <LOD | / | / | / | / | / | 199.0 | |||
| Removal | % | 81.6 | 100 | / | 96.3 | 83.1 | 78.7 | 100 | 100 | 97.6 | / | / | / | / | / | 90.2 | |||
| Primary & secondary treatment, disinfection | Influent | 2015 | ng·L−1 | 4329 | 11.7 | 2.09 | 71.8 | 119.6 | / | 0.71 | / | / | 0.14 | 0.32 | 0.62 | 0.25 | 0.78 | 4537 | [10] |
| Effluent | ng·L−1 | 548 | 5.0 | 2.04 | 8.67 | 4.74 | / | / | / | / | 0.11 | 0.22 | / | 0.16 | 0.25 | 569 | |||
| Excess sludge | ng·g−1 | 550 | 5.32 | 1.24 | 316 | 1.07 | / | / | / | / | / | 0.28 | 0.43 | 2.35 | 1.43 | 878 | |||
| Removal | % | 81 | 65 | 2 | 76 | 91 | / | / | / | / | 22 | 31 | / | 36 | 68 | 87 | |||
| Primary & secondary treatment | Influent | 2015 | ng·L−1 | 71.7 | / | / | 90.2 | 29.4 | / | / | / | / | / | / | / | / | / | 219.5 | [9] |
| Effluent | ng·L−1 | 39.1 | / | / | 65.6 | 25.5 | / | / | / | / | / | / | / | / | / | 156 | |||
| PS & SS | ng·g−1 | 599.5 | / | / | 140 | 11.8 | / | / | / | / | / | / | / | / | / | 724.5 | |||
| Removal | % | 46 | / | / | 27 | 13 | / | / | / | / | / | / | / | / | / | 29 | |||
| Primary & secondary treatment | Influent | 2015 | ng·L−1 | 4121 | 12.6 | 3.03 | 73.3 | 204 | / | 0.86 | / | / | 0.21 | 0.374 | / | <LOD | 1.31 | 4416 | [7] |
| Effluent | ng·L−1 | 267.8 | 3.35 | <LOD | 2.44 | 0.51 | / | 0.33 | / | / | <LOD | <LOD | / | <LOD | 0.68 | 275 | |||
| PS & SS | ng·g−1 | 275 | 10.2 | <LOD | 229 | 1.50 | / | / | / | <LOD | / | / | / | 3.11 | 520 | ||||
| Removal | % | 94 | 73 | / | 97 | 99 | / | 62 | / | / | / | / | / | / | 48 | 94 |
2. Materials and Methods
2.1. Standards and Reagents
2.2. Sample Collection
2.3. Experimental
2.3.1. Extraction of BPs from the Aqueous Phase
2.3.2. Extraction of BPs from the Solid Phase
Optimization of SPE (Solid Phase)
Sample Preparation: Solid Phase
2.4. Instrumental Analysis
2.5. Method Validation
2.6. Calculations of Mass Flows and BPs Removal
3. Results and Discussion
3.1. Concentrations of BPs in Different Stages of the WW Treatment Process
3.1.1. BPs in the Aqueous Phase
3.1.2. BPs in the Aqueous Phase of Sludge
3.1.3. BPs in the Solid Phase of Sludge
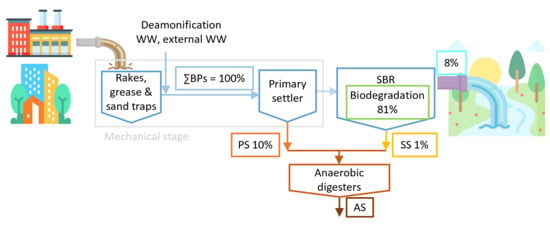
3.2. Mass Flows
3.3. Distribution and Removal of BPs from Wastewater
3.4. Removal of BPs in Anaerobic Digesters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noszczyńska, M.; Piotrowska-Seget, Z. Bisphenols: Application, Occurrence, Safety, and Biodegradation Mediated by Bacterial Communities in Wastewater Treatment Plants and Rivers. Chemosphere 2018, 201, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Lenarčič, K.; Mislej, V.; Levstek, M.; Kovačič, A.; Cimrmančič, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S.; et al. The Occurrence and Source Identification of Bisphenol Compounds in Wastewaters. Sci. Total Environ. 2018, 616–617, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A Targeted Review on Fate, Occurrence, Risk and Health Implications of Bisphenol Analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.-L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water Scarcity and Wastewater Reuse in Crop Irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Collivignarelli, M.; Abbà, A.; Frattarola, A.; Carnevale Miino, M.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Zhao, J.-L.; Zhang, C.-Y.; Rao, W.-L.; Liang, G.-H.; Zhang, H.; Liu, Y.-H.; Guan, Y.-F.; Zhang, H.-Y.; Ying, G.-G. Profile and Removal of Bisphenol Analogues in Hospital Wastewater, Landfill Leachate, and Municipal Wastewater in South China. Sci. Total Environ. 2021, 790, 148269. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Li, Y.; Ashfaq, M.; Dai, L.; Xie, X.; Yu, C.-P. Fate and Mass Balance of Bisphenol Analogues in Wastewater Treatment Plants in Xiamen City, China. Environ. Pollut. 2017, 225, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Kannan, K. Mass Flows and Removal of Eight Bisphenol Analogs, Bisphenol A Diglycidyl Ether and Its Derivatives in Two Wastewater Treatment Plants in New York State, USA. Sci. Total Environ. 2019, 648, 442–449. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.-L.; Yang, Y.-Y.; Jia, Y.-W.; Zhang, Q.-Q.; Chen, C.-E.; Liu, Y.-S.; Yang, B.; Xie, L.; Ying, G.-G. Occurrence, Mass Loads and Risks of Bisphenol Analogues in the Pearl River Delta Region, South China: Urban Rainfall Runoff as a Potential Source for Receiving Rivers. Environ. Pollut. 2020, 263, 114361. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, Fate and Risk Assessment of BPA and Its Substituents in Wastewater Treatment Plant: A Review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of Selected Pharmaceuticals and Synthetic Endocrine Disrupting Compounds during Wastewater Treatment and Sludge Anaerobic Digestion. J. Hazard. Mater. 2013, 244–245, 259–267. [Google Scholar] [CrossRef]
- Choi, Y.J.; Nies, L.F.; Lee, L.S. Persistence of Three Bisphenols and Other Trace Organics of Concern in Anaerobic Sludge under Methanogenic Conditions. Environ. Technol. 2021, 42, 1373–1382. [Google Scholar] [CrossRef]
- Phan, H.V.; Wickham, R.; Xie, S.; McDonald, J.A.; Khan, S.J.; Ngo, H.H.; Guo, W.; Nghiem, L.D. The Fate of Trace Organic Contaminants during Anaerobic Digestion of Primary Sludge: A Pilot Scale Study. Bioresour. Technol. 2018, 256, 384–390. [Google Scholar] [CrossRef]
- Abril, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Occurrence, Fate and Environmental Risk of Anionic Surfactants, Bisphenol A, Perfluorinated Compounds and Personal Care Products in Sludge Stabilization Treatments. Sci. Total Environ. 2020, 711, 135048. [Google Scholar] [CrossRef]
- Karthikraj, R.; Kannan, K. Mass Loading and Removal of Benzotriazoles, Benzothiazoles, Benzophenones, and Bisphenols in Indian Sewage Treatment Plants. Chemosphere 2017, 181, 216–223. [Google Scholar] [CrossRef]
- Kovačič, A.; Česen, M.; Laimou-Geraniou, M.; Lambropoulou, D.; Kosjek, T.; Heath, D.; Heath, E. Stability, Biological Treatment and UV Photolysis of 18 Bisphenols under Laboratory Conditions. Environ. Res. 2019, 179, 108738. [Google Scholar] [CrossRef]
- Škufca, D.; Prosenc, F.; Griessler Bulc, T.; Heath, E. Removal and Fate of 18 Bisphenols in Lab-Scale Algal Bioreactors. Sci. Total Environ. 2022, 804, 149878. [Google Scholar] [CrossRef]
- Morissette, M.-F.; Vo Duy, S.; Arp, H.P.H.; Sauvé, S. Sorption and Desorption of Diverse Contaminants of Varying Polarity in Wastewater Sludge with and without Alum. Environ. Sci. Process. Impacts 2015, 17, 674–682. [Google Scholar] [CrossRef]
- Gu, D.; Song, Z.; Kang, H.; Mao, Y.; Feng, Q. Occurrence, Profiles and Ecological Risk of Bisphenol Analogues in a Municipal Sewage Treatment Plant. Bull. Environ. Contam. Toxicol. 2021, 106, 1044–1049. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. The Quantification of Bisphenols and Their Analogues in Wastewaters and Surface Water by an Improved Solid-Phase Extraction Gas Chromatography/Mass Spectrometry Method. Environ. Sci. Pollut. Res. 2020, 27, 28829–28839. [Google Scholar] [CrossRef]
- Pivnenko, K.; Laner, D.; Astrup, T.F. Dynamics of Bisphenol A (BPA) and Bisphenol S (BPS) in the European Paper Cycle: Need for Concern? Resour. Conserv. Recycl. 2018, 133, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Liao, C.; Song, G.-J.; Ra, K.; Kannan, K.; Moon, H.-B. Emission of Bisphenol Analogues Including Bisphenol A and Bisphenol F from Wastewater Treatment Plants in Korea. Chemosphere 2015, 119, 1000–1006. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Teslic, S.; Alaee, M.; Smyth, S.A. Bisphenol-A Removal in Various Wastewater Treatment Processes: Operational Conditions, Mass Balance, and Optimization. J. Environ. Manage. 2015, 152, 192–200. [Google Scholar] [CrossRef]
- Zhu, Q.; Jia, J.; Wang, Y.; Zhang, K.; Zhang, H.; Liao, C.; Jiang, G. Spatial Distribution of Parabens, Triclocarban, Triclosan, Bisphenols, and Tetrabromobisphenol A and Its Alternatives in Municipal Sewage Sludges in China. Sci. Total Environ. 2019, 679, 61–69. [Google Scholar] [CrossRef]
- Nielsen, B.V.; Maneein, S.; Al Farid, M.M.; Milledge, J.J. The Effects of Halogenated Compounds on the Anaerobic Digestion of Macroalgae. Fermentation 2020, 6, 85. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, B.; Zhao, Y.; Zhang, J.; Shao, B. Highly Sensitive and High-Throughput Method for the Analysis of Bisphenol Analogues and Their Halogenated Derivatives in Breast Milk. J. Agric. Food Chem. 2017, 65, 10452–10463. [Google Scholar] [CrossRef]







| Compound | Mass flow PS + SS [g day−1] | Mass flow AS [g day−1] | Anaerobic Removal [%] |
|---|---|---|---|
| BPS | 0.47 | 0.33 | 28 |
| 22BPF | 0.30 | 0.07 | 78 |
| 24BPF | 0.04 | 0.45 | −1156 |
| 44BPF | 0.10 | 0.47 | −380 |
| BPE | 0.02 | 0.02 | 11 |
| BPA | 1.91 | 4.63 | −142 |
| BPAF | 0.05 | 0.01 | 80 |
| BPAP | 0.02 | 0.003 | 82 |
| BPBP | 0.01 | 0.04 | −570 |
| BPP | 0.01 | 0.003 | 67 |
| Total | 2.92 | 6.03 | −107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vehar, A.; Kovačič, A.; Hvala, N.; Škufca, D.; Levstek, M.; Stražar, M.; Žgajnar Gotvajn, A.; Heath, E. An Assessment of Mass Flows, Removal and Environmental Emissions of Bisphenols in a Sequencing Batch Reactor Wastewater Treatment Plant. Molecules 2022, 27, 8634. https://doi.org/10.3390/molecules27238634
Vehar A, Kovačič A, Hvala N, Škufca D, Levstek M, Stražar M, Žgajnar Gotvajn A, Heath E. An Assessment of Mass Flows, Removal and Environmental Emissions of Bisphenols in a Sequencing Batch Reactor Wastewater Treatment Plant. Molecules. 2022; 27(23):8634. https://doi.org/10.3390/molecules27238634
Chicago/Turabian StyleVehar, Anja, Ana Kovačič, Nadja Hvala, David Škufca, Marjetka Levstek, Marjetka Stražar, Andreja Žgajnar Gotvajn, and Ester Heath. 2022. "An Assessment of Mass Flows, Removal and Environmental Emissions of Bisphenols in a Sequencing Batch Reactor Wastewater Treatment Plant" Molecules 27, no. 23: 8634. https://doi.org/10.3390/molecules27238634