New Azido Coumarins as Potential Agents for Fluorescent Labeling and Their “Click” Chemistry Reactions for the Conjugation with closo-Dodecaborate Anion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Strategy
2.2. Synthesis
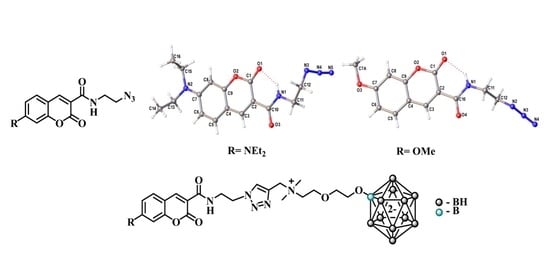
2.3. Single Crystal X-ray Diffraction Study of Azido Derivatives of Coumarins
2.4. Photophysical Properties
2.5. Antiproliferative Activity and Cell Uptake of Boronated Coumarins
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. N-(2-azidoethyl)-7-methoxy-2-oxo-2H-chromene-3-carboxamide 2a
3.1.2. N-(2-azidoethyl)-7-(diethylamino)-2-oxo-2H-chromene-3-carboxamide 2b
3.1.3. Synthesis of Conjugate 4a
3.1.4. Synthesis of Conjugates 4b
3.2. In Vitro Antiproliferative Assays and Cellular Uptake
3.3. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bansal, Y.; Sethi, P.; Bansal, G. CoumarIn A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Grovera, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Rostom, B.; Karaky, R.; Kassab, I.; Sylla-Iyarreta Veitía, M. Coumarins derivatives and inflammation: Review of their effects on the inflammatory signaling pathways. Eur. J. Pharm. 2022, 922, 174867. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Somappa, S.B. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure–activity relationship. J. Clin. Pharm. Therap. 2022, 47, 915–931. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zhang, Z.-W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, A.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, e1900380. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Mishra, S.; Pandey, A.; Manvati, S. CoumarIn An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Kong, D.; Liu, Y.; Li, M. Pharmacological perspectives and molecular mechanisms of coumarin derivatives against virus disease. Genes Deseases 2022, 9, 80–94. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.J.M.D.S.; Diederich, M. Translational role of natural coumarins and their derivatives as anticancer agents. Futur. Med. Chem. 2019, 11, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Dorababu, A. Coumarin-heterocycle framework: A privileged approach in promising anticancer drug design. Eur. J. Med. Chem. Rep. 2021, 2, 100006. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Peng, X.-M.; Damu, G.L.V.; Zhou, C.-H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Design 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Sandhu, S.; Bansal, Y.; Silakari, O.; Bansal, G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014, 22, 3806–3814. [Google Scholar] [CrossRef]
- Revankar, H.M.; Bukhari, S.N.A.; Kumar, G.B.; Qin, H.-L. Coumarins scaffolds as COX inhibitors. Bioorg. Chem. 2017, 71, 146–159. [Google Scholar] [CrossRef]
- Fotopoulos, I.; Hadjipavlou-Litina, D. Hybrids of coumarin derivatives as potent and multifunctional bioactive agents: A review. Med. Chem. 2020, 16, 272–306. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and biological properties of coumarin derivatives. A review. Chem. Select 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Katopodi, A.; Tsotsou, E.; Iliou, T.; Deligiannidou, G.-E.; Pontiki, E.; Kontogiorgis, C.; Tsopelas, F.; Detsi, A. Synthesis, bioactivity, pharmacokinetic and biomimetic properties of multi-substituted coumarin derivatives. Molecules 2021, 26, 5999. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Wang, X.; Lei, K.; Wang, S. Design, synthesis, and in vivo and in silico evaluation of coumarin derivatives with potential antidepressant effects. Molecules 2021, 26, 5556. [Google Scholar] [CrossRef]
- Koyiparambath, V.P.; Rajappan, K.P.; Rangarajan, T.M.; Al-Sehemi, A.G.; Pannipara, M.; Bhaskar, V.; Nair, A.S.; Sudevan, S.T.; Kumar, S.; Mathew, B. Deciphering the detailed structure–activity relationship of coumarins as monoamine oxidase enzyme inhibitors—An updated review. Chem. Biol. Drug Design 2021, 98, 655–673. [Google Scholar] [CrossRef]
- Agić, D.; Karnaš, M.; Šubarić, D.; Lončarić, M.; Tomić, S.; Karačić, Z.; Bešlo, D.; Rastija, V.; Molnar, M.; Popović, B.M.; et al. Coumarin derivatives act as novel inhibitors of human dipeptidyl peptidase III: Combined in vitro and in silico study. Pharmaceuticals 2021, 14, 540. [Google Scholar] [CrossRef]
- Dar’in, D.; Kantin, G.; Kalinin, S.; Sharonova, T.; Bunev, A.; Ostapenko, G.I.; Nocentini, A.; Sharoyko, V.; Supuran, C.T.; Krasavin, M. Investigation of 3-sulfamoyl coumarins against cancer-related IX and XII isoforms of human carbonic anhydrase as well as cancer cells leads to the discovery of 2-oxo-2H-benzo[h]chromene-3-sulfonamide—A new caspase-activating proapoptotic agent. Eur. J. Med. Chem. 2021, 222, 113589. [Google Scholar] [CrossRef] [PubMed]
- Balewski, L.; Szulta, S.; Jalińska, A.; Kornicka, A. A mini-review: Recent advances in coumarin-metal complexes with biological properties. Front. Chem. 2021, 9, 781779. [Google Scholar] [CrossRef]
- Patil, S.B. Medicinal significance of novel coumarin analogs: Recent studies. Results Chem. 2022, 4, 100313. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; Muncipinto, G.; Nicolotti, O.; Carrieri, A.; Rullo, M.; Stefanachi, A.; Leonetti, F.; Altomare, C. A twenty-year journey exploring coumarin-based derivatives as bioactive molecules. Front. Chem. 2022, 10, 1002547. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Cole, J.M. Molecular design of UV–vis absorption and emission properties in organic fluorophores: Toward larger bathochromic shifts, enhanced molar extinction coefficients, and greater Stokes shifts. J. Phys. Chem. C 2013, 117, 16584–16595. [Google Scholar] [CrossRef]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Martins, S.; Caldeira, A.T. Coumarins as fluorescent labels of biomolecules. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao., L., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Berthelot, T.; Talbot, J.-C.; Laïn, G.; Déleris, G.; Latxague, L. Synthesis of Nε-(7-diethylaminocoumarin-3-carboxyl)- and Nε-(7-methoxycoumarin-3-carboxyl)-L-fmoc lysine as tools for protease cleavage detection by fluorescence. J. Peptide Sci. 2005, 11, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Ngai, M.H.; Song, Z.Y.; MacAry, P.A.; Hobley, J.; Lear, M.J. Practical synthesis of maleimides and coumarin-linked probes for protein and antibody labelling via reduction of native disulfides. Org. Biomol. Chem. 2009, 7, 3400–3406. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 2012, 41, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Kohl, F.; Schmitz, J.; Furtmann, N.; Schulz-Fincke, A.-C.; Mertens, M.D.; Küppers, J.; Benkhoff, M.; Tobiasch, E.; Bartz, U.; Bajorath, J.; et al. Design, characterization and cellular uptake studies of fluorescence-labeled prototypic cathepsin inhibitors. Org. Biomol. Chem. 2015, 13, 10310–10323. [Google Scholar] [CrossRef] [PubMed]
- Häußler, D.; Gütschow, M. Fluorescently labeled amino acids as building blocks for bioactive molecules. Synthesis 2016, 48, 245–255. [Google Scholar] [CrossRef]
- Keuler, T.; Gatterdam, K.; Akbal, A.; Lovotti, M.; Marleaux, M.; Geyer, M.; Latz, E.; Gütschow, M. Development of fluorescent and biotin probes targeting NLRP3. Front. Chem. 2021, 9, 642273. [Google Scholar] [CrossRef]
- Sabnis, R.W. 7-Methoxycoumarin-3-carboxylic acid. In Handbook of Fluorescent Dyes and Probes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 281–284. [Google Scholar] [CrossRef]
- Sabnis, R.W. 7-Diethylaminocoumarin-3-carboxylic acid (DAC) (DEAC). In Handbook of Fluorescent Dyes and Probes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 149–152. [Google Scholar] [CrossRef]
- Sabnis, R.W. 7-Methoxycoumarin-3-carboxylic acid succinimidyl ester. In Handbook of Fluorescent Dyes and Probes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 285–286. [Google Scholar] [CrossRef]
- Sabnis, R.W. 7-Diethylaminocoumarin-3-carboxylic acid succinimidyl ester (DEAC SE). In Handbook of Fluorescent Dyes and Probes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 155–156. [Google Scholar] [CrossRef]
- Grayeski, M.L.; DeVasto, J.K. Coumarin derivatizing agents for carboxylic acid detection using peroxyoxalate chemiluminescence with liquid chromatography. Anal. Chem. 1987, 57, 1203. [Google Scholar] [CrossRef]
- Vemula, V.; Ni, Z.; Fedorova, M. Fluorescence labeling of carbonylated lipids and proteins in cells using coumarin-hydrazide. Redox Biol. 2015, 5, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Sabnis, R.W. 7-Diethylaminocoumarin-3-carboxylic acid hydrazide (DCCH). In Handbook of Fluorescent Dyes and Probes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 153–154. [Google Scholar] [CrossRef]
- Milic, I.; Fedorova, M. Derivatization and detection of small aliphatic and lipid-bound carbonylated lipid peroxidation products by ESI-MS. In Methods in Molecular Biology; Advanced Protocols in Oxidative Stress, III.; Armstrong, D., Ed.; Humana Press: New York, NY, USA, 2015; Volume 1208, pp. 3–20. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chio, T.I.; Bane, S.L. Visualization of oxidative stress-induced carbonylation in live mammalian cells. In Methods in Enzymology; Chemical Tools for Imaging, Manipulating, and Tracking Biological Systems: Diverse Chemical, Optical and Bioorthogonal Methods; Chenoweth, D.M., Ed.; Academic Press: Cambridge, UK, 2020; Volume 641, pp. 165–181. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, B.K. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkynecycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094. [Google Scholar] [CrossRef]
- Upadhyay, H.C. Coumarin-1,2,3-triazole hybrid molecules: An emerging scaffold for combating drug resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Musiol-Kroll, E.M.; Zubeil, F.; Schafhauser, T.; Härtner, T.; Kulik, A.; McArthur, J.; Koryakina, I.; Wohlleben, W.; Grond, S.; Williams, G.J.; et al. Polyketide bioderivatization using the promiscuous acyltransferase KirCII. ACS Synth. Biol. 2017, 6, 421–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Pelaz, B.; Chakraborty, I.; Parak, W.J. Investigating possible enzymatic degradation on polymer shells around inorganic nanoparticles. Int. J. Mol. Sci. 2019, 20, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, P.X.M.; Moroni, E.; Merlier, F.; Gheber, L.A.; Vago, R.; Tse Sum Bui, B.; Haupt, K. Chemical antibody mimics inhibit cadherin-mediated cell-cell adhesion: A promising strategy for cancer therapy. Angew. Chem. Int. Ed. Eng. 2020, 59, 2816–2822. [Google Scholar] [CrossRef]
- Soloway, A.H.; Hatanaka, H.; Davis, M.A. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. J. Med. Chem. 1967, 10, 714–717. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Derivatives of the closo-dodecaborate anion and their application in medicine. Russ. Chem. Bull. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.I.; Sivaev, I.B. Polyhedral boron compounds for BNCT. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 181–207. [Google Scholar]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-dodecaborate anion [B12H12]2−: A review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Sivaev, I.B. Polyhedral boranes and carboranes. In Comprehensive Organometallic Chemistry IV; Elsevier: Kidlington, UK, 2022; Volume 9, pp. 196–262. [Google Scholar] [CrossRef]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Kawabata, S.; Kirihata, M. Dodecaborate conjugates targeting tumor cell overexpressing translocator protein for boron neutron capture therapy. ACS Med. Chem. Lett. 2022, 13, 50–54. [Google Scholar] [CrossRef]
- Hirase, S.; Aoki, A.; Hattori, Y.; Morimoto, K.; Noguchi, K.; Fujii, I.; Takatani-Nakase, T.; Futaki, S.; Kirihata, M.; Nakase, I. Dodecaborate-encapsulated extracellular vesicles with modification of cell-penetrating peptides for enhancing macropinocytotic cellular uptake and biological activity in boron neutron capture therapy. Mol. Pharm. 2022, 19, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Koziorowski, J.; Sivaev, I.; Lundqvist, H.; Carlsson, J.; Orlova, A.; Gedda, L.; Olsson, P.; Sjöberg, S.; Sundin, A. closo-dodecaborate(2-) as a linker for iodination of macromolecules. Aspects on conjugation chemistry and biodistribution. Bioconjug. Chem. 1999, 10, 338–345. [Google Scholar] [CrossRef]
- Tolmachev, V.; Bruskin, A.; Sivaev, I.; Lundqvist, H.; Sjöberg, S. Radiobromination of closo-dodecaborate anion. Aspects of labelling chemistry in aqueous solution using Chloramine-T. Radiochim. Acta 2002, 90, 229–235. [Google Scholar] [CrossRef]
- Bruskin, A.; Sivaev, I.; Persson, M.; Lundqvist, H.; Carlsson, J.; Sjöberg, S.; Tolmachev, V. Radiobromination of monoclonal antibody using potassium [76Br](4 isothiocyanatobenzyl-ammonio)-bromo-decahydro-closo-dodecaborate (Bromo-DABI). Nucl. Med. Biol. 2004, 31, 205–211. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Chyan, M.-K.; Hamlin, D.K.; Perry, M.A. Reagents for Astatination of Biomolecules. 3. Comparison of closo-decaborate(2-) and closo-dodecaborate(2-) moieties as reactive groups for labeling with astatine-211. Bioconjug. Chem. 2009, 20, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Chaari, M.; Gaztelumendi, N.; Cabrera-González, J.; Peixoto-Moledo, P.; Viñas, C.; Xochitiotzi-Flores, E.; Farfán, N.; Ben Salah, A.; Nogués, C.; Núñez, R. Fluorescent BODIPY-anionic boron cluster conjugates as potential agents for cell tracking. Bioconjug. Chem. 2018, 29, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Justus, E.; Izteleuova, D.T.; Kasantsev, A.V.; Axartov, M.M.; Lork, E.; Gabel, D. Preparation of carboranyl and dodecaborate derivatives of coumarin. Collect. Czech. Chem. Commun. 2007, 72, 1740–1754. [Google Scholar] [CrossRef]
- Kosenko, I.; Laskova, J.; Kozlova, A.; Semioshkin, A.; Bregadze, V.I. Synthesis of coumarins modified with cobalt bis(1,2-dicarbolide) and closo-dodecaborate boron clusters. J. Organomet. Chem. 2020, 921, 121370. [Google Scholar] [CrossRef]
- Serdyukov, A.; Kosenko, I.; Druzina, A.; Grin, M.; Mironov, A.F.; Bregadze, V.I.; Laskova, J. Anionic polyhedral boron clusters conjugates with 7-diethylamino-4-hydroxycoumarin. Synthesis and lipophilicity determination. J. Organomet. Chem. 2021, 946–947, 121905. [Google Scholar] [CrossRef]
- Genady, A.R.; Ioppolo, J.A.; Azaam, M.M.; El-Zaria, M.E. New functionalized mercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. Eur. J. Med. Chem. 2015, 93, 574–583. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Semioshkin, A.A.; Brellochs, B.; Sjöberg, S.; Bregadze, V.I. Synthesis of oxonium derivatives of the dodecahydro- closo-dodecaborate anion [B12H12]2−. Tetramethylene oxonium derivative of [B12H12]2− as a convenient precursor for the synthesis of functional compounds for boron neutron capture therapy. Polyhedron 2000, 19, 627–632. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 977–992. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives as an efficient synthetic tool for the modification of polyhedral boron hydrides. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 623–637. [Google Scholar]
- Stogniy, M.Y.; Sivaev, I.B. Synthesis and reactivity of cyclic oxonium derivatives of nido-carborane: A review. Reactions 2022, 3, 172–191. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of cobalt and iron bis(dicarbollides) and their use in organic synthesis. Russ. Chem. Rev. 2021, 90, 785–830. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Semioshkin, A.A.; Las’kova, J.N.; Berzina, M.Y.; Lobanova, I.A.; Sivaev, I.B.; Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Ulybina, O.V.; et al. Novel types of boronated chlorine6 conjugates via ‘click chemistry’. Appl. Organomet. Chem. 2009, 23, 370–374. [Google Scholar] [CrossRef]
- Semioshkin, A.; Laskova, J.; Wojtczak, B.; Andrysiak, A.; Godovikov, I.; Bregadze, V.; Lesnikowski, Z.J. Synthesis of closo-dodecaborate based nucleoside conjugates. J. Organomet. Chem. 2009, 694, 1375–1379. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Nakamura, H. New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: Possible boron carriers and visualization in cells for neutron capture therapy. Inorg. Chem. 2009, 48, 11896–11902. [Google Scholar] [CrossRef]
- Koganei, H.; Tachikawa, S.; El-Zaria, M.E.; Nakamura, H. Synthesis of oligo-closo-dodecaborates by Hüisgen click reaction as encapsulated agents for the preparation of high-boron-content liposomes for neutron capture therapy. New J. Chem. 2015, 39, 6388–6394. [Google Scholar] [CrossRef]
- Goswami, L.N.; Chakravarty, S.; Lee, M.W.; Jalisatgi, S.S.; Hawthorne, M.F. Extensions of the icosahedral closomer structure by using azide–alkyne click reactions. Angew. Chem. Int. Ed. 2011, 50, 4689–4691. [Google Scholar] [CrossRef]
- Goswami, L.N.; Ma, L.; Kueffer, P.J.; Jalisatgi, S.S.; Hawthorne, M.F. Synthesis and relaxivity studies of a DOTA-based nanomolecular chelator assembly supported by an icosahedral closo-B122− -core for MRI: A click chemistry approach. Molecules 2013, 18, 9034–9048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurubuchi, T.; Shirakawa, M.; Kurosawa, W.; Matsumoto, K.; Ubagai, R.; Umishio, H.; Suga, Y.; Yamazaki, J.; Arakawa, A.; Maruyama, Y.; et al. Evaluation of a novel boron-containing α-d-mannopyranoside for BNCT. Cells 2020, 9, 1277. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Kosenko, I.D. Synthesis of conjugates of closo-dodecaborate dianion with cholesterol using a “click” reaction. Russ. Chem. Bull. 2020, 69, 1080–1084. [Google Scholar] [CrossRef]
- Novopashina, D.S.; Vorobyeva, M.A.; Lomzov, A.A.; Silnikov, V.N.; Venyaminova, A.G. Terminal mono- and bis-conjugates of oligonucleotides with closo-dodecaborate: Synthesis and physico-chemical properties. Int. J. Mol. Sci. 2021, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Druzina, A.A.; Grammatikova, N.E.; Zhidkova, O.B.; Nekrasova, N.A.; Dudarova, N.V.; Kosenko, I.D.; Grin, M.A.; Bregadze, V.I. Synthesis and antibacterial activity studies of the conjugates of curcumin with closo-dodecaborate and cobalt bis(dicarbollide) boron clusters. Molecules 2022, 27, 2920. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Kulikova, N.Y.; Nizhnik, E.A.; Vichuzhanin, M.V.; Starikova, Z.A.; Semioshkin, A.A.; Bregadze, V.I. Practical synthesis of 1,4-dioxane derivative of the closo-dodecaborate anion and its ring opening with acetylenic alkoxides. J. Organomet. Chem. 2008, 693, 519–525. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Osipov, S.N.; Grebenyuk, J.N.; Nizhnik, E.A.; Godovikov, I.A.; Shchetnikov, G.T.; Bregadze, V.I. An effective approach to 1,2,3-triazole containing 12-vertex closo-dodecaborates. Collect. Czech. Chem. Commun. 2007, 72, 1717–1724. [Google Scholar] [CrossRef]
- Prashanth, T.; Avin, B.R.V.; Thirusangu, P.; Ranganatha, V.L.; Prabhakar, B.T.; Sharath Chandra, J.N.N.; Khanum, S.A. Synthesis of coumarin analogs appended with quinoline and thiazole moiety and their apoptogenic role against murine ascitic carcinoma. Biomed. Pharm. 2019, 112, 108707. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yan, Z.; Hu, X.; Zuo, Y.; Jiang, C.; Jin, L.; Shang, Y. FeCl3-Catalyzed cascade reaction: An efficient approach to functionalized coumarin derivatives. Synth. Commun. 2014, 44, 1507–1514. [Google Scholar] [CrossRef]
- Jotani, M.M.; Baldaniya, B.B.; Tiekink, E.R.T. N-(2-Oxo-2H-chromen-3-yl)benzamide. Acta Cryst. E 2010, 66, o778. [Google Scholar] [CrossRef] [Green Version]
- Matos, M.J.; Uriarte, E.; Santana, L.; Vilar, S. Synthesis, NMR characterization, X-ray structural analysis and theoretical calculations of amide and ester derivatives of the coumarin scaffold. J. Mol. Struct. 2013, 1041, 144–150. [Google Scholar] [CrossRef]
- Mague, J.T.; Mohamed, S.K.; Akkurt, M.; Younese, S.H.H.; Albayati, M.R. Crystal structure of 2-oxo-N’-phenyl-2H-chromene- 3-carbohydrazide. Acta Cryst. E 2015, 71, o1005–o1006. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-J.; Yin, B.-Z. 7-Diethylamino-2-oxo-2H-chromene-3-carbohydrazide. Acta Cryst. E 2011, 67, o1107. [Google Scholar] [CrossRef] [Green Version]
- Olejniczak, A.; Katrusiak, A.; Podsiadło, M.; Katrusiak, A. Crystal design by CH···N and N···N interactions: High-pressure structures of high-nitrogen-content azido-triazolopyridazines compounds. Acta Cryst. B 2020, 76, 1136–1142. [Google Scholar] [CrossRef]
- Raunio, H.; Pentikainen, O.; Juvonen, R.O. Coumarin-Based Profluorescent and Fluorescent Substrates for Determining Xenobiotic-Metabolizing Enzyme Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 4708. [Google Scholar] [CrossRef]
- Titov, A.A.; Smol’yakov, A.F.; Godovikov, I.A.; Chernyadyev, A.Y.; Molotkov, A.P.; Loginov, D.A.; Filippov, O.A.; Belkova, N.V.; Shubina, E.S. The role of weak intermolecular interactions in photophysical behavior of isocoumarins on the example of their interaction with cyclic trinuclear silver(I) pyrazolate. Inorg. Chim. Acta 2022, 539, 121004. [Google Scholar] [CrossRef]
- Verlinden, B.; Van Hoecke, K.; Aerts, A.; Daems, N.; Dobney, A.; Janssens, K.; Cardinaels, T. Quantification of boron in cells for evaluation of drug agents used in boron neutron capture therapy. J. Anal. At. Spectrom. 2021, 36, 598–606. [Google Scholar] [CrossRef]
- Kasparkova, J.; Kostrhunova, H.; Novohradsky, V.; Logvinov, A.A.; Temnov, V.V.; Borisova, N.E.; Podrugina, T.A.; Markova, L.; Starha, P.; Nazarov, A.A.; et al. Novel cis-Pt(II) complexes with alkylpyrazole ligands: Synthesis, characterization, and unusual mode of anticancer action. Bioinorg. Chem. Appl. 2022, 2022, 1717200. [Google Scholar] [CrossRef] [PubMed]
- Shutkov, I.A.; Okulova, Y.N.; Tyurin, V.Y.; Sokolova, E.V.; Babkov, D.A.; Spasov, A.A.; Gracheva, Y.A.; Schmidt, C.; Kirsanov, K.I.; Shtil, A.A.; et al. Ru(III) Complexes with lonidamine-modified ligands. Int. J. Mol. Sci. 2021, 22, 13468. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, U. Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle 2009, 8, 505–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, S.-J.; Wan, Y.; Xia, Y.-Q.; Zou, X.; Zheng, S.-Y. Size-based separation methods of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 3–20. [Google Scholar] [CrossRef]
- Jiang, R.; Shen, H.; Piao, Y. The morphometrical analysis on the ultrastructure of A549 cells. Rom. J. Morphol. Embryol. 2010, 51, 663–667. [Google Scholar] [PubMed]
- Phillips, K.G.; Jacques, S.L.; McCarty, O.J.T. Measurement of single cell refractive index, dry mass, volume, and density using a transillumination microscope. Phys. Rev. Lett. 2012, 109, 118105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Antonio, M.; Biffi, G.; Mariani, A.; Raiber, E.A.; Rodriguez, R.; Balasubramanian, S. Selective RNA versus DNA G-quadruplex targeting by in situ click chemistry. Angew. Chem. Int. Ed. 2012, 124, 11235–11240. [Google Scholar] [CrossRef] [Green Version]
- Kasparkova, J.; Kostrhunova, H.; Novohradsky, V.; Ma, L.; Zhu, G.; Milaeva, E.R.; Shtill, A.A.; Vinck, R.; Gasser, G.; Brabec, V.; et al. Is antitumor Pt(IV) complex containing two axial lonidamine ligands a true dual- or multi-action prodrug? Metallomics 2022, 14, mfac048. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]








| λmaxabs (ε∙10−3, M−1 cm−1), nm | λmaxem (λex), nm | |
|---|---|---|
| 2a | 350 (5.0) | 398 (340) |
| 4a | 343 (13.4) | 405 (340) |
| 2b | 413 (9.1) | 464 (410) |
| 4b | 430 (12.6) | 474 (410) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskova, J.; Serdyukov, A.; Kosenko, I.; Ananyev, I.; Titova, E.; Druzina, A.; Sivaev, I.; Antonets, A.A.; Nazarov, A.A.; Bregadze, V.I. New Azido Coumarins as Potential Agents for Fluorescent Labeling and Their “Click” Chemistry Reactions for the Conjugation with closo-Dodecaborate Anion. Molecules 2022, 27, 8575. https://doi.org/10.3390/molecules27238575
Laskova J, Serdyukov A, Kosenko I, Ananyev I, Titova E, Druzina A, Sivaev I, Antonets AA, Nazarov AA, Bregadze VI. New Azido Coumarins as Potential Agents for Fluorescent Labeling and Their “Click” Chemistry Reactions for the Conjugation with closo-Dodecaborate Anion. Molecules. 2022; 27(23):8575. https://doi.org/10.3390/molecules27238575
Chicago/Turabian StyleLaskova, Julia, Alexander Serdyukov, Irina Kosenko, Ivan Ananyev, Ekaterina Titova, Anna Druzina, Igor Sivaev, Anastasia A. Antonets, Alexey A. Nazarov, and Vladimir I. Bregadze. 2022. "New Azido Coumarins as Potential Agents for Fluorescent Labeling and Their “Click” Chemistry Reactions for the Conjugation with closo-Dodecaborate Anion" Molecules 27, no. 23: 8575. https://doi.org/10.3390/molecules27238575