Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Synthesis and Characterization
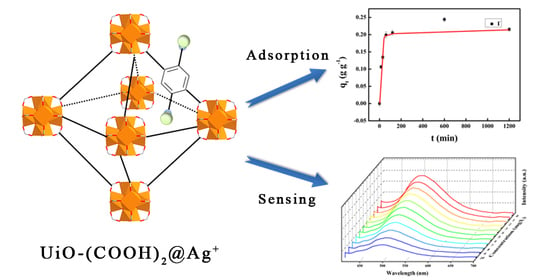
2.2. Iodide Adsorption
2.3. Sensing Properties
3. Conclusions
4. Materials and Methods
4.1. Synthesis of UiO-66-(COOH)2
4.2. Preparation of Ag+@UiO-66-(COOH)2
4.3. Analytical Methods and Characterization
4.4. Iodides Capture Studies
4.5. Iodides Concentration Dependent Luminescence Spectra
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Atomic Energy Agency. Energy, Electricity and Nuclear Power Estimates for the Period Up to 2050; International Atomic Energy Agency: Vienna, Austria, 2015; Volume 37. [Google Scholar]
- Gu, J.-M.; Kim, S.-J.; Kim, Y.; Huh, S. Structural isomerism of an anionic nanoporous In-MOF with interpenetrated diamond-like topology. CrystEngComm 2012, 14, 1819. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Han, S.; Um, W.; Kim, W.-S. Development of bismuth-functionalized graphene oxide to remove radioactive iodine. Dalton Trans. 2019, 48, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Zhang, M.; Cao, K.; Tian, Y.; Qi, Y.; Li, S.; Li, K.; Yu, X.; Ma, L. Colyliform Crystalline 2D Covalent Organic Frameworks (COFs) with Quasi-3D Topologies for Rapid I2 Adsorption. Angew. Chem. Int. Ed. 2020, 59, 22697–22705. [Google Scholar] [CrossRef]
- Xu, S.; Freeman, S.P.H.T.; Hou, X.; Watanabe, A.; Yamaguchi, K.; Zhang, L. Iodine Isotopes in Precipitation: Temporal Responses to 129I Emissions from the Fukushima Nuclear Accident. Environ. Sci. Technol. 2013, 47, 10851–10859. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, M.; Tokonami, S.; Tazoe, H.; Sorimachi, A.; Monzen, S.; Osanai, M.; Akata, N.; Kakiuchi, H.; Omori, Y.; Ishikawa, T.; et al. Activity concentrations of environmental samples collected in Fukushima Prefecture immediately after the Fukushima nuclear accident. Sci. Rep. 2013, 3, 2283. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, Z.; Ma, X.; Li, S.; Gong, R.; Kuang, A. Targeting of tumor radioiodine therapy by expression of the sodium iodide symporter under control of the survivin promoter. Cancer Gene Therapy 2011, 18, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.S.; Swanson, R.L.; Cochran, J.K. Medically-derived 131I in municipal sewage effluent. Water Res. 2012, 46, 5663–5671. [Google Scholar] [CrossRef]
- Rose, P.S.; Smith, J.P.; Cochran, J.K.; Aller, R.C.; Swanson, R.L. Behavior of medically-derived 131I in the tidal Potomac River. Sci. Total Environ. 2013, 452–453, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Povinec, P.P.; Zhang, L.; Shi, K.; Biddulph, D.; Chang, C.-C.; Fan, Y.; Golser, R.; Hou, Y.; Ješkovsk, M.; et al. Iodine-129 in Seawater Offshore Fukushima_ Distribution, Inorganic Speciation, Sources, and Budget. Environ. Sci. Technol. 2013, 47, 3091–9098. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Takahashi, Y. Selective immobilization of iodide onto a novel bismuth-impregnated layered mixed metal oxide_ Batch and EXAFS studies. J. Hazard. Mater. 2020, 384, 121223. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef]
- Mu, W.; Yu, Q.; Li, X.; Wei, H.; Jian, Y. Niobate nanofibers for simultaneous adsorptive removal of radioactive strontium and iodine from aqueous solution. J. Alloys Compd. 2017, 693, 550–557. [Google Scholar] [CrossRef]
- Mao, P.; Qi, L.; Liu, X.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Synthesis of Cu_Cu2O hydrides for enhanced removal of iodide from water. J. Hazard. Mater. 2017, 328, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, P.; Li, X.; Zhang, G. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O_Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Zhao, X.; Chen, D.; Cai, R.; Yang, W.; Komarneni, S.; Yang, D. Selective Capture of Iodide from Solutions by Microrosette-like δ-Bi2O3. ACS Appl. Mater. Interfaces 2014, 6, 16082–16090. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, S.; Wang, H.; Wang, G.; Zhao, H.; Cai, W. Nanosheets-built flowerlike micro_nanostructured Bi2O2.33 and its highly efficient iodine removal performances. Chem. Eng. J. 2016, 289, 219–230. [Google Scholar] [CrossRef]
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized Ionic Porous Organic Polymers Exhibiting High Iodine Uptake from Both the Vapor and Aqueous Medium. ACS Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef]
- Mao, P.; Qi, B.; Liu, Y.; Zhao, L.; Jiao, Y.; Zhang, Y.; Jiang, Z.; Li, Q.; Wang, J.; Chen, S.; et al. AgII doped MIL-101 and its adsorption of iodine with high speed in solution. J. Solid State Chem. 2016, 237, 274–283. [Google Scholar] [CrossRef]
- Wan, J.; Li, Y.; Jiang, Y.; Lin, L.; Yin, Y. Silver-doped MIL-101(Cr) for rapid and effective capture of iodide in water environment_ exploration on adsorption mechanism. J. Radioanal. Nucl. Chem. 2021, 328, 1041–1054. [Google Scholar] [CrossRef]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal–Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Shen, N.; Yang, Z.; Liu, S.; Dai, X.; Xiao, C.; Taylor-Pashow, K.; Li, D.; Yang, C.; Li, J.; Zhang, Y.; et al. 99TcO4− removal from legacy defense nuclear waste by an alkaline-stable 2D cationic metal organic framework. Nat. Commun. 2020, 11, 5571. [Google Scholar] [CrossRef]
- Chen, M.; Liu, T.; Zhang, X.; Zhang, R.; Tang, S.; Yuan, Y.; Xie, Z.; Liu, Y.; Wang, H.; Fedorovich, K.V.; et al. Photoinduced Enhancement of Uranium Extraction from Seawater by MOF_Black Phosphorus Quantum Dots Heterojunction Anchored on Cellulose Nanofiber Aerogel. Adv. Func. Mater. 2021, 31, 2100106. [Google Scholar] [CrossRef]
- Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2020, 427, 213473. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore Chemistry of Metal–Organic Frameworks. Adv. Funct. Mater. 2020, 30, 2000238. [Google Scholar] [CrossRef]
- Zhao, X.; Han, X.; Li, Z.; Huang, H.; Liu, D.; Zhong, C. Enhanced removal of iodide from water induced by a metal-incorporated porous metal–organic framework. Appl. Surf. Sci. 2015, 351, 760–764. [Google Scholar] [CrossRef]
- Rio, M.d.; Villar, M.; Quesada, S.; Palomino, G.T.; Ferrer, L.; Cabello, C.P. Silver-functionalized UiO-66 metal-organic framework-coated 3D printed device for the removal of radioactive iodine from wastewaters. Appl. Mater. Today 2021, 24, 101130. [Google Scholar]
- Xu, W.; Zhang, W.; Kang, J.; Li, B. Facile synthesis of mesoporous Fe-based MOFs loading bismuth with high speed adsorption of iodide from solution. J. Solid State Chem. 2019, 269, 558–565. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shojaie, A.F.; Montazerozohori, M.; Karami, B.; Gharaghani, S. Iodide-Selective Electrodes Based on Bis[N(2-methyl-phenyl) 4-Nitro-thiobenzamidato]mercury(II) and Bis[N-phenyl 3,5-Dinitro-thiobenzamidato]mercury(II) Carriers. Electroanalysis 2005, 19, 1746–1754. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Willander, M. A Selective Iodide Ion Sensor Electrode Based on Functionalized ZnO Nanotubes. Sensors 2013, 13, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Terufumi, F.; Mohammadzai, I.U.; Inoue, H.; Takahiro, K. Chemiluminescence determination of iodide and/or iodine using a luminol-hexadecyltrimethylammonium chloride reversed micelle system following on-line oxidation and extraction. Analyst 2000, 125, 759–763. [Google Scholar]
- Yang, G.-L.; Jiang, X.-L.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mei, Q.; Yu, L.; Ge, H.; Yue, J.; Zhang, K.; Hayat, T.; Alsaedi, A.; Wang, S. Rapid and On-Site Detection of Uranyl Ions via Ratiometric Fluorescence Signals Based on a Smartphone Platform. ACS Appl. Mater. Interfaces 2018, 10, 42225–42232. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Zhang, D.; Liang, C.; Liu, W.; Chong, Y.; Yin, X.; Zhang, Y.; Gui, D.; Chen, L.; et al. Photo-exfoliation of a highly photo-responsive two-dimensional metal–organic framework. Chem. Commun. 2019, 55, 11715–11718. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Shao, Z.; Yan, X.; Liu, M.; Yu, L.; Wan, Y.; Chang, D.; Zhang, J.; Zhao, D. Tailoring the triplet level of isomorphic Eu_Tb mixed MOFs for sensitive temperature sensing. Chem. Commun. 2021, 57, 3143–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, E.; Liu, F.; Li, H.; Xia, T. Growth of robust metal-organic framework films by spontaneous oxidation of a metal substrate for NO2 sensing. Mater. Chem. Front. 2021, 5, 6476–6484. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Xia, T.; Zhang, J.; Yang, Y.; Cui, Y.; Chen, B.; Qian, G. Turn-on and Ratiometric Luminescent Sensing of Hydrogen Sulfide Based on Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2016, 8, 32259–32265. [Google Scholar] [CrossRef]
- Wu, D.; Maurin, G.; Yang, Q.; Serre, C.; Jobic, H.; Zhong, C. Computational exploration of a Zr-carboxylate based metal-organic framework as a membrane material for CO2 capture. J. Mater. Chem. A 2014, 2, 1657–1661. [Google Scholar] [CrossRef]
- Guillerm, V.; Gross, S.; Serre, C.; Devic, T.; Bauer, M.; Ferey, G. A zirconium methacrylate oxocluster as precursor for the low-temperature synthesis of porous zirconium(IV) dicarboxylates. Chem. Commun. 2010, 46, 767–769. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, L.; Jiang, K.; He, H.; Yang, Y.; Cui, Y.; Li, B.; Qian, G. Nanoscale fluorescent metal–organic framework composites as a logic platform for potential diagnosis of asthma. Biosens. Bioelectron. 2019, 130, 65–72. [Google Scholar] [CrossRef]
- Mao, P.; Liu, Y.; Jiao, Y.; Chen, S.; Yang, Y. Enhanced uptake of iodide on Ag@Cu2O nanoparticles. Chemosphere 2016, 164, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Zhao, Z.; Jing, Z.; Zhang, T.; Qiu, F.; Xu, J. High-specifc surface area hierarchical Al2O3 carbon fber based on a waste paper fber template: Preparation and adsorption for iodide ions. J. Wood Chem. Technol. 2017, 37, 485–492. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Z.; Yang, J.; Wang, G.; Wang, H.; Cai, H. Au25(SG)18 as a fluorescent iodide sensor. Nanoscale 2012, 4, 4087–4090. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, X.; Wang, Z.; Mu, S.; Liao, J.; Wen, Y.; Lv, J.; Huang, K.; Xiong, X. A sensitive and label-free sensor for melamine and iodide by target-regulating the formation of G-quadruplex. Microchem. J. 2019, 146, 592–599. [Google Scholar]
- Dang, Q.; Wan, H.; Zhan, X. Carbazolic porous framework with tetrahedral core for gas uptake and tandem detection of iodide and mercury. ACS Appl. Mater. Interfaces 2017, 9, 21438–21446. [Google Scholar] [CrossRef]
- Salomón-Flores, M.; Hernández-Juárez, C.; Bazany-Rodríguez, I.; Barroso-Flores, J.; Martínez-Otero, D.; López-Arteaga, R.; Valdés-Martínez, J.; Dorazco-González, A. Efficient fluorescent chemosensing of iodide based on a cationic meso-tetraarylporphyrin in pure water. Sens. Actuators B 2019, 281, 462–470. [Google Scholar] [CrossRef]
- Singha, D.; Majee, P.; Mondal, S.; Mahata, P. Luminescent cadmium based MOF as selective and sensitive iodide sensor in aqueous medium. J. Photochem. Photobiol. A 2018, 356, 389–396. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, R.; Feng, S.; Wang, D.; Liu, H. Porosity-induced selective sensing of iodide in aqueous solution by a fluorescent imidazolium-based ionic porous framework. ACS Appl. Mater. Interfaces 2020, 12, 11104–11114. [Google Scholar] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, S.; Shao, L.; Ren, Y.; Jiang, J.; Wang, H.; Tang, H.; Deng, H.; Xia, T. Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework. Molecules 2022, 27, 8547. https://doi.org/10.3390/molecules27238547
Zhang J, Yang S, Shao L, Ren Y, Jiang J, Wang H, Tang H, Deng H, Xia T. Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework. Molecules. 2022; 27(23):8547. https://doi.org/10.3390/molecules27238547
Chicago/Turabian StyleZhang, Jun, Shanli Yang, Lang Shao, Yiming Ren, Jiaolai Jiang, Huaisheng Wang, Hao Tang, Hui Deng, and Tifeng Xia. 2022. "Highly Sensitive Adsorption and Detection of Iodide in Aqueous Solution by a Post-Synthesized Zirconium-Organic Framework" Molecules 27, no. 23: 8547. https://doi.org/10.3390/molecules27238547