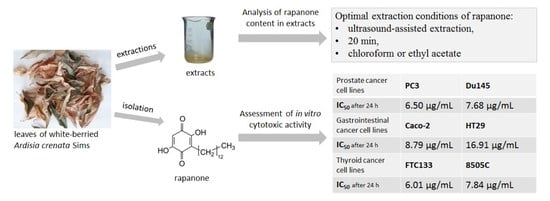
Optimization of Extraction Conditions and Cytotoxic Activity of Rapanone in Comparison to Its Homologue, Embelin
Abstract
:1. Introduction
2. Results
2.1. Optimization of Rapanone Extraction from Plant Material
2.2. Cytotoxic Activity of Rapanone and Embelin
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Plant Material
4.3. Optimization of Extraction Conditions and Preparation of Samples and Study Design
4.4. Quantitative HPLC Analysis
4.5. Extraction, Isolation, and Identification of the Benzoquinones
4.6. Cell Culture and In Vitro Cytotoxic Assay
- Asample is the absorbance value for cells treated with the tested substances
- Aspont is the value for spontaneous LDH release
- Amax the value in cells lysed in the presence of Triton X100
- The half-maximal effective concentration value IC50, defined as the substance concentration necessary to obtain 50% of dead cells, was determined.
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Podolak, I.; Mynarski, A.; Wróbel, D.; Grabowska, K.; Galanty, A. Bioactive benzoquinones content variability in red-berry and white-berry varieties of Ardisia crenata Sims. and assessment of cytotoxic activity. Nat. Prod. Res. 2019, 35, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Podolak, I.; Strzałka, M. Qualitative and quantitative LC profile of embelin and rapanone in selected Lysimachia species. Chromatographia 2008, 67, 471–475. [Google Scholar] [CrossRef]
- Calle, J.; Olarte, J.; Pinzon, R.; Ospina, L.F.; Mendoza, M.C.; Orozco, M.J. Alterations in the reproduction of mice induced by rapanone. J. Ethnopharmacol. 2000, 71, 521–525. [Google Scholar] [CrossRef]
- Lal, B.; Mishra, N. Importance of Embelia ribes: An update. Int. J. Pharm. Sci. Res. 2013, 4, 3823. [Google Scholar]
- Souravi, K.; Rajasekharan, P.E. Ethnopharmacological uses of Embelia ribes Burm. F. A review. IOSR J. Pharm. Biol. Sci. 2014, 9, 23–30. [Google Scholar] [CrossRef]
- Kundap, U.P.; Bhuvanendran, S.; Kumari, Y.; Othman, I.; Shaikh, M.F. Plant derived phytocompound, embelin in CNS disorders: A systematic review. Front. Pharmacol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, J.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Sethi, G.; Mishra, S.; Shanmugam, M.K.; Ahn, K.S. The application of embelin for cancer prevention and therapy. Molecules 2018, 23, 621. [Google Scholar] [CrossRef] [Green Version]
- Othman, S.N.N.; Lum, P.T.; Sekar, M.; Mazlan, N.A.; Yusri, P.Z.S.; Ghazali, N.F.; Mohd, I.M.; Shazalyana, A.; Ismail, M.; Noor, A.A.M. Molecules of interest–embelin—A review. Res. J. Pharm. Technol. 2020, 13, 3485–3493. [Google Scholar] [CrossRef]
- Nikolovska-Coleska, Z.; Xu, L.; Hu, Z.; Tomita, Y.; Li, P.; Roller, P.P.; Wang, R.; Fang, X.; Guo, R.; Zhang, M.; et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 2004, 47, 2430–2440. [Google Scholar] [CrossRef]
- Ospina, L.F.; Calle, J.; Arteaga, L.; Pinzón, R.; Alcaraz, M.J.; Payá, M. Inhibition of acute and chronic inflammatory responses by the hydroxybenzoquinonic derivative rapanone. Planta Med. 2001, 67, 791–795. [Google Scholar] [CrossRef]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Żmudzki, P.; Podolak, I. Anti-melanoma potential of two benzoquinone homologues embelin and rapanone-a comparative in vitro study. Toxicol. Vitr. 2020, 65, 104826. [Google Scholar] [CrossRef]
- Cordero, C.P.; Gómez-González, S.; León-Acosta, C.J.; Morantes-Medina, S.J.; Aristizabal, F.A. Cytotoxic activity of five compounds isolated from Colombian plants. Fitoterapia 2004, 75, 225–227. [Google Scholar] [CrossRef]
- Kuete, V.; Omosa, L.K.; Tala, V.R.S.; Midiwo, J.O.; Mbaveng, A.T.; Swaleh, S.; Karaosmanoglu, O.; Sivas, H. Cytotoxicity of plumbagin, rapanone and 12 other naturally occurring quinones from Kenyan flora towards human carcinoma cells. BMC Pharm. Toxicol. 2016, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Andreu, G.L.P.; Dos Reis, F.Z.; González-Durruthy, M.; Hernández, R.D.; D'Vries, R.F.; Berghe, W.V.; Alberici, L.C. Rapanone, a naturally occurring benzoquinone, inhibits mitochondrial respiration and induces HepG2 cell death. Toxicol. Vitr. 2020, 63, 104737. [Google Scholar] [CrossRef]
- de la Vega-Hernández, K.; Antuch, M.; Cuesta-Rubio, O.; Núñez-Figueredo, Y.; Pardo-Andreu, G.L. Discerning the antioxidant mechanism of rapanone: A naturally occurring benzoquinone with iron complexing and radical scavenging activities. J. Inorg. Biochem. 2017, 170, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Kiprono, P.C. Chemistry and Some Biological Activities of Benzoquinones of Embelia schimperi. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 1997. [Google Scholar]
- Ganesan, B.; Perumal, P.; Manickam, V.; Gotteti, S.D.; Srikakolapu, S.R.; Thirumurthy, L.S. Optimization of extraction conditions for embelin in Embelia ribes by UV spectrophotometry. Arch. App. Sci. Res. 2010, 2, 49–53. [Google Scholar]
- Alam, M.S.; Damanhouri, Z.A.; Ahmad, A.; Abidin, L.; Amir, M.; Aqil, M.; Khan, S.A.; Mujeeb, M. Development of response surface methodology for optimization of extraction parameters and quantitative estimation of embelin from Embelia ribes Burm by high performance liquid chromatography. Pharm. Mag 2015, 11, 166. [Google Scholar]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 29 October 2022).
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Mahendran, S.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine 2011, 18, 186–188. [Google Scholar] [CrossRef]
- Thippeswamy, B.S.; Mahendran, S.; Biradar, M.I.; Raj, P.; Srivastava, K.; Badami, S.; Veerapur, V.P. Protective effect of embelin against acetic acid induced ulcerative colitis in rats. Eur. J. Pharm. 2011, 654, 100–105. [Google Scholar] [CrossRef]
- Kaur, V.; Hallan, S.S.; Nidhi, A.N.; Mishra, N. Isolation of embelin from and evaluation of its anti-cancer potential in Embelia ribes breast cancer. Asian J. Pharm. Pharm. 2015, 1, 33–39. [Google Scholar]
- Durg, S.; Kumar, N.; Vandal, R.; Dhadde, S.B.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Antipsychotic activity of embelin isolated from Embelia ribes: A preliminary study. Biomed. Pharm. 2017, 90, 328–331. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; Sortino, M.; Zacchino, S.; de Arias, A.R.; Inchausti, A.; Yaluff, G.; Rodriguez, J.; Theoduloz, C.; Schmeda-Hirschmann, G. Bioactive alkyl phenols and embelin from Oxalis erythrorhiza. J. Ethnopharmacol. 2003, 88, 241–247. [Google Scholar] [CrossRef]
- Swamy, H.K.; Krishna, V.; Shankarmurthy, K.; Rahiman, B.A.; Mankani, K.L.; Mahadevan, K.M.; Harish, B.G.; Naika, H.R. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J. Ethnopharmacol. 2007, 109, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Gupta, G.; Kazmi, I.; Rahman, M.; Upadhyay, G.; Ahmad, K.; Imam, F.; Pravez, M.; Anwar, F. Evaluation of anxiolytic activity of embelin isolated from Embelia ribes. Biomed. Aging Pathol. 2012, 2, 45–47. [Google Scholar] [CrossRef]
- Gupta, G.; Kazmi, I.; Afzal, M.; Upadhyay, G.; Singh, R.; Habtemariam, S. Antidepressant-like activity of Embelin isolated from Embelia ribes. Phytopharmacology 2013, 4, 87–95. [Google Scholar]
- da Costa, R.C.; Santana, D.B.; Araújo, R.M.; de Paula, J.E.; do Nascimento, P.C.; Lopes, N.P.; Braz-Filho, R.; Espindola, L.S. Discovery of the rapanone and suberonone mixture as a motif for leishmanicidal and antifungal applications. Bioorg. Med. Chem. 2014, 22, 135–140. [Google Scholar] [CrossRef]
- Reguero, M.T.; Mata, R. De la Corteza de Rapanea guíanensís Aubl Det. wordsck. Rev. Colomb. Cien. Quím.-Farm. 1989, 17, 17–23. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Akhtar, S.; Khan, A.Q.; Siveen, K.S.; Uddin, S. Embelin: A benzoquinone possesses therapeutic potential for the treatment of human cancer. Future Med. Chem. 2018, 10, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Devi Daimary, U.; Girisa, S.; Parama, D.; Verma, E.; Kumar, A.; Kunnumakkara, A.B. Embelin: A novel XIAP inhibitor for the prevention and treatment of chronic diseases. J. Biochem. Mol. Toxicol. 2022, 36, e22950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ning, L.; Wang, L.; Ouyang, T.; Qi, L.; Yang, R.; Wu, Y. miR-21 inhibition reverses doxorubicin-resistance and inhibits PC3 human prostate cancer cells proliferation. Andrologia 2021, 53, e14016. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Singh, R.P.; Agarwal, C.; Chan, D.C.; Agarwal, R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res. 2002, 8, 3512–3519. [Google Scholar] [PubMed]
- Massart, C.; Poirier, C.; Fergelot, P.; Fardel, O.; Gibassier, J. Effect of sodium butyrate on doxorubicin resistance and expression of multidrug resistance genes in thyroid carcinoma cells. Anti-Cancer Drugs 2005, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- El-Zawahry, A.; McKillop, J.; Voelkel-Johnson, C. Doxorubicin increases the effectiveness of Apo2L/TRAIL for tumor growth inhibition of prostate cancer xenografts. BMC Cancer 2005, 5, 1–9. [Google Scholar] [CrossRef]
- Xu, T.; Li, T.; Zhang, Y.; Huan, S.; Cui, T.; Sun, L. Inhibition of Akt/NF-κB/survivin pathway by embelin on castration-resistant prostate cancer cells. Int. J. Clin. Exp. Med. 2017, 10, 4386–4397. [Google Scholar]
- Park, N.; Baek, H.S.; Chun, Y.J. Embelin-induced apoptosis of human prostate cancer cells is mediated through modulation of Akt and β-Catenin signaling. PLoS ONE 2015, 10, e0134760. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.-H.; Ahn, K.S.; Choi, S.-H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Heo, J.Y.; Kim, H.J.; Kim, S.M.; Park, K.R.; Park, S.Y.; Kim, S.W.; Nam, D.; Jang, H.-J.; Lee, S.-G.; Ahn, K.S.; et al. Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, W.B.; Yim, J.H.; Kim, W.G.; Kim, T.Y.; Choi, H.J.; Kim, E.Y.; Shong, Y.K. The effect of a XIAP inhibitor, embelin, on apoptosis of thyroid cancer cell lines. Cancer Res. 2012, 72, 1709. [Google Scholar] [CrossRef]
- Hussain, A.R.; Bu, R.; Ahmed, M.; Jehan, Z.; Beg, S.; Al-Sobhi, S.; Al-Dayel, F.; Siraj, A.K.; Uddin, S.; Al-Kuraya, K.S. Role of X-linked inhibitor of apoptosis as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2015, 100, E974–E985. [Google Scholar] [CrossRef] [Green Version]
- Sumalatha, K.; Gowda, M.; Meenakshisundaram, S. ROS-mediated induction of apoptosis by benzoquinone embelin in human colon adenocarcinoma cells HT-29. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Janeczko, Z. Cytotoxic activity of embelin from Lysimachia punctata. Fitoterapia 2005, 76, 333–335. [Google Scholar] [CrossRef]
- Grabowska, K.; Podolak, I.; Galanty, A.; Żmudzki, P.; Koczurkiewicz, P.; Piska, K.; Pękala, E.; Janeczko, Z. Two new triterpenoid saponins from the leaves of Impatiens parviflora DC. and their cytotoxic activity. Ind. Crop. Prod. 2017, 96, 71–79. [Google Scholar] [CrossRef]
- Shoemaker, J. Robust Outlier Identification Using SAS® . Available online: https://support.sas.com/resources/papers/proceedings/proceedings/sugi24/Infovis/p161-24.pdf (accessed on 15 September 2022).



| Set Code | HRE | Set Code | SE | Set Code | UAE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | Time of Extraction [min] | Number of Extraction Repetitions | Solvent | Time of Extraction [min] | Number of Extraction Repetitions | Solvent | Time of Extraction [min] | Number of Extraction Repetitions | |||
| 1H | chloroform | 30 | 1 | 1S | chloroform | 30 | 1 | 1U | chloroform | 10 | 1 |
| 2H | chloroform | 30 | 2 | 2S | chloroform | 30 | 2 | 2U | chloroform | 10 | 2 |
| 3H | chloroform | 30 | 3 | 3S | chloroform | 30 | 3 | 3U | chloroform | 10 | 3 |
| 4H | chloroform | 60 | 1 | 4S | chloroform | 60 | 1 | 4U | chloroform | 20 | 1 |
| 5H | chloroform | 60 | 2 | 5S | chloroform | 60 | 2 | 5U | chloroform | 20 | 2 |
| 6H | chloroform | 60 | 3 | 6S | chloroform | 60 | 3 | 6U | chloroform | 20 | 3 |
| 7H | chloroform | 120 | 1 | 7S | chloroform | 120 | 1 | 7U | chloroform | 30 | 1 |
| 8H | chloroform | 120 | 2 | 8S | chloroform | 120 | 2 | 8U | chloroform | 30 | 2 |
| 9H | chloroform | 120 | 3 | 9S | chloroform | 120 | 3 | 9U | chloroform | 30 | 3 |
| 10H | ethyl acetate | 30 | 1 | 10S | ethyl acetate | 30 | 1 | 10U | ethyl acetate | 10 | 1 |
| 11H | ethyl acetate | 30 | 2 | 11S | ethyl acetate | 30 | 2 | 11U | ethyl acetate | 10 | 2 |
| 12H | ethyl acetate | 30 | 3 | 12S | ethyl acetate | 30 | 3 | 12U | ethyl acetate | 10 | 3 |
| 13H | ethyl acetate | 60 | 1 | 13S | ethyl acetate | 60 | 1 | 13U | ethyl acetate | 20 | 1 |
| 14H | ethyl acetate | 60 | 2 | 14S | ethyl acetate | 60 | 2 | 14U | ethyl acetate | 20 | 2 |
| 15H | ethyl acetate | 60 | 3 | 15S | ethyl acetate | 60 | 3 | 15U | ethyl acetate | 20 | 3 |
| 16H | ethyl acetate | 120 | 1 | 16S | ethyl acetate | 120 | 1 | 16U | ethyl acetate | 30 | 1 |
| 17H | ethyl acetate | 120 | 2 | 17S | ethyl acetate | 120 | 2 | 17U | ethyl acetate | 30 | 2 |
| 18H | ethyl acetate | 120 | 3 | 18S | ethyl acetate | 120 | 3 | 18U | ethyl acetate | 30 | 3 |
| 19H | acetone | 30 | 1 | 19S | acetone | 30 | 1 | 19U | acetone | 10 | 1 |
| 20H | acetone | 30 | 2 | 20S | acetone | 30 | 2 | 20U | acetone | 10 | 2 |
| 21H | acetone | 30 | 3 | 21S | acetone | 30 | 3 | 21U | acetone | 10 | 3 |
| 22H | acetone | 60 | 1 | 22S | acetone | 60 | 1 | 22U | acetone | 20 | 1 |
| 23H | acetone | 60 | 2 | 23S | acetone | 60 | 2 | 23U | acetone | 20 | 2 |
| 24H | acetone | 60 | 3 | 24S | acetone | 60 | 3 | 24U | acetone | 20 | 3 |
| 25H | acetone | 120 | 1 | 25S | acetone | 120 | 1 | 25U | acetone | 30 | 1 |
| 26H | acetone | 120 | 2 | 26S | acetone | 120 | 2 | 26U | acetone | 30 | 2 |
| 27H | acetone | 120 | 3 | 27S | acetone | 120 | 3 | 27U | acetone | 30 | 3 |
| Set Code | HRE | Set Code | SE | Set Code | UAE | |||
|---|---|---|---|---|---|---|---|---|
| Mean [mg/g] | Median [mg/g] | Mean [mg/g] | Median [mg/g] | Mean [mg/g] | Median [mg/g] | |||
| 1H | 1.69 ± 0.17 | 1.70 | 1S | 2.23 ± 0.14 | 2.24 | 1U | 17.42 ± 1.38 | 17.29 |
| 2H | 1.86 ± 0.20 | 1.92 | 2S | 2.75 ± 0.03 | 2.76 | 2U | 17.93 ± 0.51 | 18.01 |
| 3H | 1.72 ± 0.15 | 1.70 | 3S | 2.45 ± 0.35 | 2.54 | 3U | 19.77 ± 1.30 | 20.15 |
| 4H | 1.70 ± 0.32 | 1.61 | 4S | 2.32 ± 0.28 | 2.22 | 4U | 20.54 ± 0.91 | 20.18 |
| 5H | 1.73 ± 0.30 | 1.71 | 5S | 2.83 ± 0.33 | 2.75 | 5U | 19.48 ± 1.10 | 19.25 |
| 6H | 1.83 ± 0.33 | 1.74 | 6S | 3.06 ± 0.32 | 2.93 | 6U | 17.54 ± 1.22 | 17.19 |
| 7H | 2.15 ± 0.21 | 2.16 | 7S | 2.30 ± 0.14 | 3.06 | 7U | 20.09 ± 1.63 | 21.11 |
| 8H | 2.53 ± 0.71 | 2.37 | 8S | 2.99 ± 0.27 | 3.03 | 8U | 17.16 ± 1.63 | 16.86 |
| 9H | 2.00 ± 0.20 | 2.01 | 9S | 3.28 ± 0.47 | 3.20 | 9U | 17.86 ± 0.54 | 17.88 |
| 10H | 11.39 ± 0.90 | 11.52 | 10S | 11.76 ± 1.08 | 11.82 | 10U | 12.21 ± 3.09 | 12.30 |
| 11H | 11.16 ± 5.08 | 9.37 | 11S | 18.42 ± 3.86 | 19.27 | 11U | 12.99 ± 3.79 | 11.82 |
| 12H | 10.33 ± 1.86 | 10.80 | 12S | 17.79 ± 0.22 | 17.85 | 12U | 13.06 ± 1.39 | 13.29 |
| 13H | 13.18 ± 0.45 | 13.28 | 13S | 7.16 ± 0.93 | 7.05 | 13U | 21.39 ± 1.21 | 21.06 |
| 14H | 14.10 ± 1.74 | 13.91 | 14S | 11.90 ± 1.08 | 12.24 | 14U | 19.40 ± 3.41 | 19.36 |
| 15H | 12.92 ± 1.97 | 12.39 | 15S | 12.76 ± 1.89 | 12.75 | 15U | 14.74 ± 2.29 | 14.70 |
| 16H | 14.80 ± 1.65 | 15.09 | 16S | 8.11 ± 1.25 | 8.68 | 16U | 18.28 ± 1.37 | 18.04 |
| 17H | 14.18 ± 1.27 | 14.46 | 17S | 6.43 ± 1.28 | 6.86 | 17U | 18.71 ± 2.46 | 17.99 |
| 18H | 17.00 ± 2.67 | 16.42 | 18S | 8.34 ± 2.69 | 8.41 | 18U | 10.71 ± 2.13 | 10.77 |
| 19H | 2.68 ± 1.05 | 2.43 | 19S | 0.05 ± 0.05 | 0.06 | 19U | 3.34 ± 0.31 | 3.27 |
| 20H | 2.79 ± 1.56 | 2.83 | 20S | 0.25 ± 0.27 | 0.15 | 20U | 2.92 ± 0.66 | 3.07 |
| 21H | 2.75 ± 0.88 | 2.26 | 21S | 0.02 ± 0.01 | 0.02 | 21U | 2.18 ± 0.71 | 2.23 |
| 22H | 2.05 ± 0.80 | 2.10 | 22S | 0.13 ± 0.13 | 0.07 | 22U | 0.04 ± 0.05 | 0.01 |
| 23H | 4.40 ± 2.46 | 4.12 | 23S | 0.14 ± 0.06 | 0.117 | 23U | 0.10 ± 0.09 | 0.04 |
| 24H | 2.24 ± 0.85 | 2.55 | 24S | Nd | Nd | 24U | Nd | Nd |
| 25H | 2.50 ± 0.81 | 2.14 | 25S | 0.11 ± 0.06 | 0.12 | 25U | 0.17 ± 0.12 | 0.11 |
| 26H | 1.97 ± 1.22 | 1.61 | 26S | 0.01 ± 0.02 | 0.000 | 26U | 0.01 ± 0.01 | 0.00 |
| 27H | 1.35 ± 0.77 | 1.49 | 27S | Nd | Nd | 27U | 0.10 ± 0.09 | 0.09 |
| IC50 [μg/mL] | ||||||
|---|---|---|---|---|---|---|
| Compound | PC3 | Du145 | PNT2 | |||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Rapanone | 6.50 | 4.92 | 7.68 | 5.07 | 16.72 | 12.65 |
| Embelin | 9.27 | 5.24 | 8.02 | 4.18 | 10.14 | 4.61 |
| Doxorubicin | >50.00 | NE | 3.18 | NE | 1.38 | NE |
| IC50 [μg/mL] | ||||||
|---|---|---|---|---|---|---|
| Compound | Caco-2 | HT29 | HepG2 | |||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Rapanone | 8.79 | 5.66 | 16.91 | 11.67 | 36.27 | 16.70 |
| Embelin | 6.12 | 3.37 | 24.70 | 13.72 | 15.98 | 6.77 |
| Doxorubicin | 3.44 | NE | 1.53 | NE | 1.03 | NE |
| IC50 [μg/mL] | ||||
|---|---|---|---|---|
| Compound | FTC133 | 8505C | ||
| 24 h | 48 h | 24 h | 48 h | |
| Rapanone | 6.01 | 4.42 | 7.84 | 5.50 |
| Embelin | 10.51 | 6.48 | 18.86 | 13.84 |
| Doxorubicin | 4.02 | NE | >40.00 | NE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Biedrawa, D.; Galanty, A.; Zagrodzki, P.; Podolak, I. Optimization of Extraction Conditions and Cytotoxic Activity of Rapanone in Comparison to Its Homologue, Embelin. Molecules 2022, 27, 7912. https://doi.org/10.3390/molecules27227912
Wróbel-Biedrawa D, Galanty A, Zagrodzki P, Podolak I. Optimization of Extraction Conditions and Cytotoxic Activity of Rapanone in Comparison to Its Homologue, Embelin. Molecules. 2022; 27(22):7912. https://doi.org/10.3390/molecules27227912
Chicago/Turabian StyleWróbel-Biedrawa, Dagmara, Agnieszka Galanty, Paweł Zagrodzki, and Irma Podolak. 2022. "Optimization of Extraction Conditions and Cytotoxic Activity of Rapanone in Comparison to Its Homologue, Embelin" Molecules 27, no. 22: 7912. https://doi.org/10.3390/molecules27227912