Chemical Composition, Antioxidant Activity, Cytoprotective and In Silico Study of Ethanolic Extracts of Bougainvillea × buttiana (Var. Orange and Rose)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material Collection and Extraction
2.2.1. Plant Material Collection
2.2.2. Extraction Procedures
2.2.3. Analysis of Total Phenolic Contents
2.2.4. GC-MS Analysis for Characterization
2.3. Measurement of Antioxidant Activity
2.3.1. ORAC Oxygen Radical Absorbance Capacity
2.3.2. DPPH Radicals Scavenging Activity
2.3.3. Antioxidant Activity NO (Nitric Oxide) Assay
2.4. Cell Proliferation and Viability Assay
2.5. Hydrogen Peroxide-Induced Oxidative Stress in L929 Cells and Evaluation of Survival
2.6. In Silico Analysis of the Compounds
2.7. Statistical Analyses
3. Results
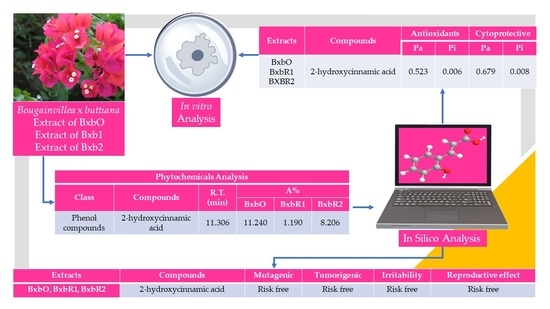
3.1. Phytocompounds
3.2. Total Phenolic Contents in Bougainvillea Extracts
3.3. Comparative Antioxidant Activity
3.4. Effect of Extracts on Nitric Oxide Capture
3.5. Effect of Extracts on Cell Viability and Proliferation
3.6. Hydrogen Peroxide-Induced Oxidative Stress and Survival
3.7. In Silico Analysis of the Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative Stress and Antioxidant Mechanisms in Human Body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Sundaram Sanjay, S.; Shukla, A.K. Free Radicals Versus Antioxidants. In Potential Therapeutic Applications of Nano-Antioxidants; Springer: Singapore, 2021; pp. 1–17. [Google Scholar]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative Stress in Biological Systems and Its Relation with Pathophysiological Functions: The Effect of Physical Activity on Cellular Redox Homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Sundaram Sanjay, S.; Shukla, A.K. Mechanism of Antioxidant Activity. In Potential Therapeutic Applications of Nano-Antioxidants; Springer: Singapore, 2021; pp. 83–99. [Google Scholar]
- Radi, R. Oxygen Radicals, Nitric Oxide, and Peroxynitrite: Redox Pathways in Molecular Medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological Chemistry of Superoxide Radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Ford, P.C.; Miranda, K.M. The Solution Chemistry of Nitric Oxide and Other Reactive Nitrogen Species. Nitric Oxide 2020, 103, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 1042. [Google Scholar] [CrossRef] [PubMed]
- Carlström, M. Nitric Oxide Signalling in Kidney Regulation and Cardiometabolic Health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, H.-J.; Oh, H.-T.; Chung, M.-J.; Cui, C.-B.; Ham, S.-S. Cytoprotective Effect by Antioxidant Activity of Codonopsis lanceolata and Platycodon grandiflorum Ethyl Acetate Fraction in Human HepG2 Cells. Korean J. Food Sci. Technol. 2008, 40, 696–701. [Google Scholar]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric Oxide and Its Derivatives in the Cancer Battlefield. Nitric oxide 2019, 93, 102–114. [Google Scholar] [CrossRef]
- Compaore, M.; Bakasso, S.; Meda, R.N.T.; Nacoulma, O.G. Antioxidant and Anti-Inflammatory Activities of Fractions from Bidens engleri O.E. Schulz (Asteraceae) and Boerhavia erecta L. (Nyctaginaceae). Medicines 2018, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Saleem, H.; Htar, T.T.; Naidu, R.; Zengin, G.; Ahmad, I.; Ahemad, N. Phytochemical Profiling, Antioxidant, Enzyme Inhibition and Cytotoxic Potential of Bougainvillea glabra Flowers. Nat. Prod. Res. 2020, 34, 2602–2606. [Google Scholar] [CrossRef]
- Alvarez Perez Gil, A.L.; Barbosa Navarro, L.; Patipo Vera, M.; Petricevich, V.L. Anti-Inflammatory and Antinociceptive Activities of the Ethanolic Extract of Bougainvillea × buttiana. J. Ethnopharmacol. 2012, 144, 712–719. [Google Scholar] [CrossRef]
- Arteaga Figueroa, L.; Abarca-Vargas, R.; García Alanis, C.; Petricevich, V.L. Comparison between Peritoneal Macrophage Activation by Bougainvillea × buttiana Extract and LPS and/or Interleukins. Biomed Res. Int. 2017, 2017, 4602952. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.K.R.; Andrade, E.H.A.; Guimaraes, E.F.; Maia, J.G.S. Essential Oil Composition, Antioxidant Capacity and Antifungal Activity of Piper divaricatum. Nat. Prod. Commun. 2010, 5, 1934578X1000500327. [Google Scholar] [CrossRef] [Green Version]
- Abarca-Vargas, R.; Peña Malacara, C.; Petricevich, V. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea × buttiana Holttum and Standl, (Var. Rose) Extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC− Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Akkari, H.; Hajaji, S.; B’chir, F.; Rekik, M.; Gharbi, M. Correlation of Polyphenolic Content with Radical-Scavenging Capacity and Anthelmintic Effects of Rubus ulmifolius (Rosaceae) against Haemonchus Contortus. Vet. Parasitol. 2016, 221, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. Nitrite Reduction by Molybdoenzymes: A New Class of Nitric Oxide-Forming Nitrite Reductases. JBIC J. Biol. Inorg. Chem. 2015, 20, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Balekar, N.; Jain, D.K.; Dixit, P.V.; Bhadoriya, S.S. Evaluation of Antiulcer Activity of Whole Plant Extract of Malvastrum tricuspidatum in Experimental Animals. Iran. J. Pharmacol. Ther. 2012, 11, 53–59. [Google Scholar]
- Marvin MarvinSketch 22.13 2022. Available online: http://www.chemaxon.com (accessed on 1 August 2022).
- PASSonline. Available online: http://www.way2drug.com/passonline/predict.php (accessed on 1 August 2022).
- Sander, T. OSIRIS Property Explorer. Available online: https://www.organic-chemistry.org/prog/peo/ (accessed on 1 August 2022).
- Arteaga Figueroa, L.; Barbosa Navarro, L.; Patino Vera, M.; Petricevich, V.L. Preliminary Studies of the Immunomodulator Effect of the Bougainvillea × buttiana Extract in a Mouse Model. Evid. Based. Complement. Alternat. Med. 2015, 2015, 479412. [Google Scholar] [CrossRef] [Green Version]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea Genus: A Review on Phytochemistry, Pharmacology, and Toxicology. Evidence-Based Complement. Altern. Med. eCAM 2018, 2018, 9070927. [Google Scholar] [CrossRef] [Green Version]
- Villanueva Guerrero, R.; Abarca-Vargas, R.; Petricevich, V.L. Chemical Compounds and Biological Activity of an Extract from Bougainvillea × buttiana (Var. Rose) Holttum and Standl. Int J Pharm Pharm Sci 2017, 9, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [Green Version]
- Engwa, G.A.; EnNwekegwa, F.N.; Nkeh-Chungag, B.N. Free Radicals, Oxidative Stress-Related Diseases and Antioxidant Supplementation. Altern. Ther. Health Med. 2022, 28, 114–128. [Google Scholar] [PubMed]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular Matrix Metabolites as Potential Biomarkers of Disease Activity in Wound Fluid: Lessons Learned from Other Inflammatory Diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Fellegrini, N.; Ke, R.; Yang, M.; Rice-Evans, C. Screening of Dietary Carotenoids and Carotenoid-Rich Fruit Extracts for Antioxidant Activities Applying 2, 2′-Azinobis (3-Ethylenebenzothiazoline-6-Sulfonic Acid Radical Cation Decolorization Assay. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 379–389. ISBN 0076-6879. [Google Scholar]
- Leite-Legatti, A.V.; Batista, Â.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Machado, A.R.T.; de Carvalho-Silva, L.B.; Ruiz, A.L.T.G. Jaboticaba peel: Antioxidant Compounds, Antiproliferative and Antimutagenic Activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Batista, Â.G.; Lenquiste, S.A.; Cazarin, C.B.B.; da Silva, J.K.; Luiz-Ferreira, A.; Bogusz, S., Jr.; Hantao, L.W.; de Souza, R.N.; Augusto, F.; Prado, M.A. Intake of Jaboticaba peel Attenuates Oxidative Stress in Tissues and Reduces Circulating Saturated Lipids of Rats with High-Fat Diet-Induced Obesity. J. Funct. Foods 2014, 6, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.K.; Guo, Q.; Packer, L. Free Radical Scavenging Activity of Red Ginseng Aqueous Extracts. Toxicology 2002, 172, 149–156. [Google Scholar] [CrossRef]
- de Castro, V.C.; da Silva, P.H.A.; de Oliveira, E.B.; Desobry, S.; Humeau, C. Extraction, Identification and Enzymatic Synthesis of Acylated Derivatives of Anthocyanins from Jaboticaba (Myrciaria cauliflora) Fruits. Int. J. food Sci. Technol. 2014, 49, 196–204. [Google Scholar] [CrossRef]
- Gangwar, M.; Gautam, M.K.; Ghildiyal, S.; Nath, G.; Goel, R.K. Mallotus Philippinensis Muell. Arg Fruit Glandular Hairs Extract Promotes Wound Healing on Different Wound Model in Rats. BMC Complement. Altern. Med. 2015, 15, 123. [Google Scholar] [CrossRef] [Green Version]
- Möller, M.N.; Cuevasanta, E.; Orrico, F.; Lopez, A.C.; Thomson, L.; Denicola, A. Diffusion and Transport of Reactive Species across Cell Membranes. In Bioactive Lipids in Health and Disease; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–19. [Google Scholar]
- Fulda, S.; Vucic, D. Targeting IAP Proteins for Therapeutic Intervention in Cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef]
- Waligórska-Stachura, J.; Jankowska, A.; Waśko, R.; Liebert, W.; Biczysko, M.; Czarnywojtek, A.; Baszko-Błaszyk, D.; Shimek, V.; Ruchała, M. Survivin–Prognostic Tumor Biomarker in Human Neoplasms–Review. Ginekol. Pol. 2012, 83, 537–540. [Google Scholar]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and Indirect Antioxidant Properties of Inducers of Cytoprotective Proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Reports 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, R. Efficacy of Trans-2-Hydroxycinnamic acid against Trichlorfon-Induced Oxidative Stress in Wistar Rats. Toxicol. Int. 2012, 19, 295–300. [Google Scholar] [CrossRef] [PubMed]







| Class | Compounds | R.T. (min) | Area% | ||
|---|---|---|---|---|---|
| BxbO | BxbR1 | BxbR2 | |||
| Pyran | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 10.293 | 5.867 | ||
| Phenol compounds | 2-Hydroxycinnamic acid | 11.306 | 11.240 | 1.190 | 8.206 |
| Benzofuran | 2,3-Dihydro-1-benzofuran | 11.621 | 15.469 | ||
| Phenol compounds | 2-Methoxy-4-vinylphenol | 11.925 | 0.224 | 5.588 | |
| Carbohydrate | 3-O-methyl-D-glucose | 16.292 | 11.192 | 92.135 | 47.924 |
| Fatty acid | Tetradecanoic acid | 17.369 | 10.765 | ||
| Hydrocarbon | 1-Nonadecene | 17.750 | 10.726 | ||
| Fatty ester | Methyl palmitate | 19.096 | 5.535 | ||
| Fatty acid | n-Hexadecanoic acid | 19.445 | 11.522 | 0.760 | |
| Fatty ester | Hexadecanoic acid, ethyl ester | 19.656 | 1.171 | 5.654 | |
| Fatty ester | Isopropyl palmitate | 20.082 | 10.985 | ||
| Fatty ester | 9,12-Octadecadienoic acid, ethyl ester | 21.246 | 1.933 | 5.757 | |
| Fatty ester | Ethyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | 21.311 | 2.587 | ||
| Ester | Diisopropyl maleate | 21.967 | 11.258 | ||
| Phthalate ester | 1,2-Benzenedicarboxylic acid, diisooctyl ester | 25.692 | 11.237 | ||
| Terpene | Squalene | 29.981 | 11.075 | ||
| Extract | Total Phenolic Contents, mgGA */dry Extract |
|---|---|
| BxbO | 27.43 ± 0.03 |
| BxbR1 | 29.55 ± 0.05 |
| BxbR2 | 32.48 ± 0.08 |
| Extract | DPPH µMTE/100 g Dry Extract | ORAC Value µM Trolox Equivalent TE/g |
|---|---|---|
| BxbO | 1478.20 ± 100.80 | 7114.43 ± 781.48 |
| BxbR1 | 1684.63 ± 146.37 | 8100.45 ± 798.85 |
| BxbR2 | 1802.54 ± 158.46 | 8105.38 ± 787.92 |
| Extracts | Compounds | Antioxidant | Cytoprotective | ||
|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | ||
| BxbR2 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 0.587 | 0.005 | 0.652 | 0.013 |
| BxbO, BxbR1, BxbR2 | 2-Hydroxycinnamic acid | 0.523 | 0.006 | 0.679 | 0.008 |
| BxbR2 | 2,3-Dihydro-1-benzofuran | 0.176 | 0.073 | 0.482 | 0.068 |
| BxbR1, BxbR2 | 2-Methoxy-4-vinylphenol | 0.459 | 0.008 | 0.735 | 0.004 |
| BxbO, BxbR1, BxbR2 | 3-O-methyl-D-glucose | - | - | 0.352 | 0.130 |
| BxbO | Tetradecanoic acid | 0.222 | 0.045 | 0.712 | 0.004 |
| BxbO | 1-Nonadecene | 0.282 | 0.027 | 0.655 | 0.013 |
| BxbR2 | Methyl palmitate | 0.210 | 0.050 | 0.701 | 0.005 |
| BxBO, BxbR1 | n-Hexadecanoic acid | 0.222 | 0.045 | 0.712 | 0.004 |
| BxbR1, BxbR2 | Hexadecanoic acid, ethyl ester | 0.201 | 0.055 | 0.715 | 0.004 |
| BxbO | Isopropyl palmitate | 0.227 | 0.043 | 0.654 | 0.013 |
| BxbR1, BxbR2 | 9,12-Octadecadienoic acid, ethyl ester | 0.285 | 0.026 | 0.720 | 0.004 |
| BxbR1 | Ethyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate | 0.320 | 0.020 | 0.704 | 0.005 |
| BxbO | Diisopropyl maleate | 0.378 | 0.014 | 0.618 | 0.024 |
| BxbO | 1,2-Benzenedicarboxylic acid, diisooctyl ester | 0.174 | 0.075 | 0.512 | 0.059 |
| BxbO | Squalene | 0.657 | 0.004 | 0.552 | 0.047 |
| Extracts | Compounds | M | T | I | R.E. |
|---|---|---|---|---|---|
| BxbR2 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one |  |  |  |  |
| BxbO, BxbR1, BxbR2 | 2-Hydroxycinnamic acid |  |  |  |  |
| BxbR2 | 2,3-Dihydro-1-benzofuran |  |  |  |  |
| BxbR1, BxbR2 | 2-Methoxy-4-vinylphenol |  |  |  |  |
| BxbO, BxbR1, BxbR2 | 3-O-methyl-D-glucose |  |  |  |  |
| BxbO | Tetradecanoic acid |  |  |  |  |
| BxbO | 1-Nonadecene |  |  |  |  |
| BxbR2 | Methyl palmitate |  |  |  |  |
| BxbO, BxbR1 | n-Hexadecanoic acid |  |  |  |  |
| BxbR1, BxbR2 | Hexadecanoic acid, ethyl ester |  |  |  |  |
| BxbO | Isopropyl palmitate |  |  |  |  |
| BxbR1, BxbR2 | 9,12-Octadecadienoic acid, ethyl ester |  |  |  |  |
| BxbR1 | Ethyl (9Z,12Z,15Z)-octadeca-9,12,15-trienoate |  |  |  |  |
| BxbO | Diisopropyl maleate |  |  |  |  |
| BxbO | 1,2-Benzenedicarboxylic acid, diisooctyl ester |  |  |  |  |
| BxbO | Squalene |  |  |  |  |
 : Risk free,
: Risk free,  : Medium risk,
: Medium risk,  : High risk., BxbO (Orange), BxbR1 (Rose1) and BxbR2 (Rose2)
: High risk., BxbO (Orange), BxbR1 (Rose1) and BxbR2 (Rose2)Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petricevich, V.L.; Cedillo-Cortezano, M.; Abarca-Vargas, R. Chemical Composition, Antioxidant Activity, Cytoprotective and In Silico Study of Ethanolic Extracts of Bougainvillea × buttiana (Var. Orange and Rose). Molecules 2022, 27, 6555. https://doi.org/10.3390/molecules27196555
Petricevich VL, Cedillo-Cortezano M, Abarca-Vargas R. Chemical Composition, Antioxidant Activity, Cytoprotective and In Silico Study of Ethanolic Extracts of Bougainvillea × buttiana (Var. Orange and Rose). Molecules. 2022; 27(19):6555. https://doi.org/10.3390/molecules27196555
Chicago/Turabian StylePetricevich, Vera L., Mayra Cedillo-Cortezano, and Rodolfo Abarca-Vargas. 2022. "Chemical Composition, Antioxidant Activity, Cytoprotective and In Silico Study of Ethanolic Extracts of Bougainvillea × buttiana (Var. Orange and Rose)" Molecules 27, no. 19: 6555. https://doi.org/10.3390/molecules27196555