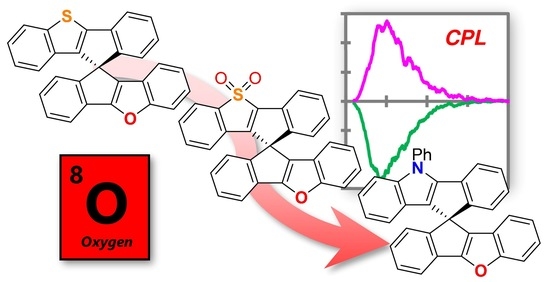
Furan-Containing Chiral Spiro-Fused Polycyclic Aromatic Compounds: Synthesis and Photophysical Properties
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
3.2.1. Methyl 2-(1-Benzofuran-2-yl)benzoate (5)

3.2.2. rac-Spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene] (rac-1)

3.2.3. 2-(2-Bromo-4-methoxyphenyl)-1-benzothiophene (7)

3.2.4. 2-(1-Benzofuran-2-yl)benzoic Acid (13)
3.2.5. 10H-Indeno[1,2-b][1]benzofuran-10-one (8)
3.2.6. rac-2′-Methoxyspiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene] (rac-10)
3.2.7. rac-Spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene]-2′-ol (rac-11)
3.2.8. rac-Spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene]-2′-yl Trifluoromethanesulfonate (rac-12)
3.2.9. rac-Spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene] (rac-1)
3.2.10. rac-Spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b][1]benzothiophene] 5′,5′-Dioxide (rac-2)
3.2.11. rac-5′-Phenyl-5′H-spiro[indeno[1,2-b][1]benzofuran-10,10′-indeno[1,2-b]indole] (rac-3)
3.3. Computational Studies
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pudzich, R.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro compounds for organic electroluminescence and related applications. Adv. Polym. Sci. 2006, 199, 83–142. [Google Scholar] [CrossRef]
- Saragi, T.P.I.; Spehr, T.; Siebert, A.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro Compounds for Organic Optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xia, D.; Baumgarten, M. Rigidly Fused Spiro-Conjugated π-Systems. ChemPlusChem 2021, 86, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ohshita, J.; Lee, K.-H.; Hamamoto, D.; Kunugi, Y.; Ikadai, J.; Kwak, Y.-W.; Kunai, A. Synthesis of Novel Spiro-condensed Dithienosiloles and the Application to Organic FET. Chem. Lett. 2004, 33, 892–893. [Google Scholar] [CrossRef]
- Saragi, T.P.I.; Fuhrmann-Lieker, T.; Salbeck, J. Comparison of Charge-Carrier Transport in Thin Films of Spiro-Linked Compounds and Their Corresponding Parent Compounds. Adv. Funct. Mater. 2006, 16, 966–974. [Google Scholar] [CrossRef]
- Poriel, C.; Rault-Berthelot, J. Structure–property relationship of 4-substituted-spirobifluorenes as hosts for phosphorescent organic light emitting diodes: An overview. J. Mater. Chem. C 2017, 5, 3869–3897. [Google Scholar] [CrossRef]
- Poriel, C.; Sicard, L.; Rault-Berthelot, J. New generations of spirobifluorene regioisomers for organic electronics: Tuning electronic properties with the substitution pattern. Chem. Commun. 2019, 55, 14238–14254. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.-K.; Zheng, Q.; Fan, J.; Liao, L.-S.; Jiang, Z.-Q. Spiro Compounds for Organic Light-Emitting Diodes. Acc. Mater. Res. 2021, 2, 1261–1271. [Google Scholar] [CrossRef]
- Ma, S.; Fu, Y.; Ni, D.; Mao, J.; Xie, Z.; Tu, G. Spiro-fluorene based 3D donor towards efficient organic photovoltaics. Chem. Commun. 2012, 48, 11847–11849. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, Y.; Zheng, Y.-Q.; Pei, J.; Zhao, D. Towards rational design of organic electron acceptors for photovoltaics: A study based on perylenediimide derivatives. Chem. Sci. 2013, 4, 4389. [Google Scholar] [CrossRef]
- Wu, X.-F.; Fu, W.-F.; Xu, Z.; Shi, M.; Liu, F.; Chen, H.-Z.; Wan, J.-H.; Russell, T.P. Spiro Linkage as an Alternative Strategy for Promising Nonfullerene Acceptors in Organic Solar Cells. Adv. Funct. Mater. 2015, 25, 5954–5966. [Google Scholar] [CrossRef]
- Yi, J.; Wang, Y.; Luo, Q.; Lin, Y.; Tan, H.; Wang, H.; Ma, C.Q. A 9,9′-spirobi[9H-fluorene]-cored perylenediimide derivative and its application in organic solar cells as a non-fullerene acceptor. Chem. Commun. 2016, 52, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Ooyama, Y.; Higashimura, H.; Ohshita, J. Synthesis, Properties, and Polymerization of Spiro[(dipyridinogermole)(dithienogermole)]. Organometallics 2015, 35, 20–26. [Google Scholar] [CrossRef]
- Ohshita, J.; Hayashi, Y.; Adachi, Y.; Enoki, T.; Yamaji, K.; Ooyama, Y. Optical and Photosensitizing Properties of Spiro(dipyridinogermole)(dithienogermole)s with Eletron-Donating Amino and Electron-Withdrawing Pyridinothiadiazole Substituents. ChemistrySelect 2018, 3, 8604–8609. [Google Scholar] [CrossRef]
- Ohshita, J.; Kondo, K.; Adachi, Y.; Song, M.; Jin, S.-H. Synthesis of spirodithienogermole with triphenylamine units as a dopant-free hole-transporting material for perovskite solar cells. J. Mater. Chem. C 2021, 9, 2001–2007. [Google Scholar] [CrossRef]
- Terada, N.; Uematsu, K.; Higuchi, R.; Tokimaru, Y.; Sato, Y.; Nakano, K.; Nozaki, K. Synthesis and Properties of Spiro-double Sila[7]helicene: The LUMO Spiro-conjugation. Chem. Eur. J. 2021, 27, 9342–9349. [Google Scholar] [CrossRef] [PubMed]
- Dobler, M.; Miljnko, D.; Martin, E.; Vladmir, P.; Dumić, M.; Egli, M.; Prelog, V. Chiral Poly(9,9′-spirobifIuorene) Crown Ethers. Angew. Chem. Int. Ed. 1985, 24, 792–794. [Google Scholar] [CrossRef]
- Alcazar, V.; Diederich, F. Enantioselective Complexation of Chiral Dicarboxylic Acids in Clefts of Functionalized 9,9′-Spirobifluorenes. Angew. Chem. Int. Ed. 1992, 31, 1521–1523. [Google Scholar] [CrossRef]
- Hovorka, R.; Meyer-Eppler, G.; Piehler, T.; Hytteballe, S.; Engeser, M.; Topic, F.; Rissanen, K.; Lützen, A. Unexpected Self-Assembly of a Homochiral Metallosupramolecular M4L4 Catenane. Chem. Eur. J. 2014, 20, 13253–13258. [Google Scholar] [CrossRef]
- Hovorka, R.; Hytteballe, S.; Piehler, T.; Meyer-Eppler, G.; Topic, F.; Rissanen, K.; Engeser, M.; Lützen, A. Self-assembly of metallosupramolecular rhombi from chiral concave 9,9′-spirobifluorene-derived bis(pyridine) ligands. Beilstein J. Org. Chem. 2014, 10, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Xie, J.H.; Li, S.; Zhou, Q.L. Asymmetric hydrogenation of α,β-unsaturated carboxylic acids catalyzed by ruthenium(II) complexes of spirobifluorene diphosphine (SFDP) ligands. Adv. Synth. Catal. 2006, 348, 1271–1276. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Q.; Xie, J.-H.; Wang, L.-X.; Zhou, Q.-L. Highly Rigid Diphosphane Ligands with a Large Dihedral Angle Based on a Chiral Spirobifluorene Backbone. Angew. Chem. Int. Ed. 2005, 44, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Murai, M.; Takeuchi, Y.; Yamauchi, K.; Kuninobu, Y.; Takai, K. Rhodium-Catalyzed Synthesis of Chiral Spiro-9-silabifluorenes by Dehydrogenative Silylation: Mechanistic Insights into the Construction of Tetraorganosilicon Stereocenters. Chem. Eur. J. 2016, 22, 6048–6058. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Inoue, Y.; Mori, T. Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation between Excitation and Emission Dissymmetry Factors. ChemPhotoChem 2018, 2, 386–402. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Yan, B. Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules. Molecules 2018, 23, 3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.L.; Peng, Q.; Zhao, C.H. Circularly Polarized Luminescence Switching in Small Organic Molecules. Chem. Eur. J. 2019, 25, 15441–15454. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. The Progress of Circularly Polarized Luminescence in Chiral Purely Organic Materials. Adv. Photonics Res. 2021, 2, 2000136. [Google Scholar] [CrossRef]
- Wan, S.-P.; Lu, H.-Y.; Li, M.; Chen, C.-F. Advances in circularly polarized luminescent materials based on axially chiral compounds. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100500. [Google Scholar] [CrossRef]
- Shintani, R.; Misawa, N.; Takano, R.; Nozaki, K. Rhodium-Catalyzed Synthesis and Optical Properties of Silicon-Bridged Arylpyridines. Chem. Eur. J. 2017, 23, 2660–2665. [Google Scholar] [CrossRef]
- Miki, K.; Noda, T.; Gon, M.; Tanaka, K.; Chujo, Y.; Mizuhata, Y.; Tokitoh, N.; Ohe, K. Near-Infrared Circularly Polarized Luminescence through Intramolecular Excimer Formation of Oligo(p-phenyleneethynylene)-Based Double Helicates. Chem. Eur. J. 2019, 25, 9211–9216. [Google Scholar] [CrossRef]
- Feng, J.; Fu, L.; Geng, H.; Jiang, W.; Wang, Z. Designing a near-infrared circularly polarized luminescent dye by dissymmetric spiro-fusion. Chem. Commun. 2019, 56, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Oniki, J.; Moriuchi, T.; Kamochi, K.; Tobisu, M.; Amaya, T. Linear [3]Spirobifluorenylene: An S-Shaped Molecular Geometry of p-Oligophenyls. J. Am. Chem. Soc. 2019, 141, 18238–18245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Kamochi, K.; Kodama, T.; Tobisu, M.; Amaya, T. Chiral cyclic [n]spirobifluorenylenes: Carbon nanorings consisting of helically arranged quaterphenyl rods illustrating partial units of woven patterns. Chem. Sci. 2020, 11, 9604–9610. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wang, Y.K.; Peng, C.C.; Wu, Z.G.; Yuan, S.; Yu, Y.J.; Li, H.; Wang, T.T.; Li, H.C.; Zheng, Y.X.; et al. Circularly Polarized Thermally Activated Delayed Fluorescence Emitters in Through-Space Charge Transfer on Asymmetric Spiro Skeletons. J. Am. Chem. Soc. 2020, 142, 17756–17765. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Nakamuro, T.; Yamashita, K.; Yanagisawa, H.; Nureki, O.; Kikkawa, M.; Harano, K.; Shang, R.; Nakamura, E. Spiro-Conjugated Carbon/Heteroatom-Bridgedp-Phenylenevinylenes: Synthesis, Properties, and Microcrystal Electron Crystallographic Analysis of Racemic Solid Solutions. Bull. Chem. Soc. Jpn. 2020, 93, 776–782. [Google Scholar] [CrossRef]
- Hamada, H.; Itabashi, Y.; Shang, R.; Nakamura, E. Axially Chiral Spiro-Conjugated Carbon-Bridged p-Phenylenevinylene Congeners: Synthetic Design and Materials Properties. J. Am. Chem. Soc. 2020, 142, 2059–2067. [Google Scholar] [CrossRef]
- Yang, S.Y.; Feng, Z.Q.; Fu, Z.; Zhang, K.; Chen, S.; Yu, Y.J.; Zou, B.; Wang, K.; Liao, L.S.; Jiang, Z.Q. Highly Efficient Sky-Blue π-Stacked Thermally Activated Delayed Fluorescence Emitter with Multi-Stimulus Response Properties. Angew. Chem. Int. Ed. 2022, 61, in press. [Google Scholar] [CrossRef]
- Takase, K.; Noguchi, K.; Nakano, K. Circularly Polarized Luminescence from Chiral Spiro Molecules: Synthesis and Optical Properties of 10,10′-Spirobi(indeno[1,2-b][1]benzothiophene) Derivatives. Org. Lett. 2017, 19, 5082–5085. [Google Scholar] [CrossRef]
- Takase, K.; Noguchi, K.; Nakano, K. [1]Benzothiophene-Fused Chiral Spiro Polycyclic Aromatic Compounds: Optical Resolution, Functionalization, and Optical Properties. J. Org. Chem. 2018, 83, 15057–15065. [Google Scholar] [CrossRef]
- Takase, K.; Noguchi, K.; Nakano, K. Synthesis of Pyrrole-Containing Chiral Spiro Molecules and Their Optical and Chiroptical Properties. Bull. Chem. Soc. Jpn. 2019, 92, 1008–1017. [Google Scholar] [CrossRef]
- Kubo, M.; Takase, K.; Noguchi, K.; Nakano, K. Solvent-sensitive circularly polarized luminescent compounds bearing a 9,9′-spirobi[fluorene] skeleton. Org. Biomol. Chem. 2020, 18, 2866–2876. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Noguchi, K.; Nakano, K. Chiral Benzo[b]silole-Fused 9,9′-Spirobi[fluorene]: Synthesis, Chiroptical Properties, and Transformation to pi-Extended Polycyclic Arene. ChemPlusChem 2021, 86, 171–175. [Google Scholar] [CrossRef]
- Bulumulla, C.; Gunawardhana, R.; Gamage, P.L.; Miller, J.T.; Kularatne, R.N.; Biewer, M.C.; Stefan, M.C. Pyrrole-Containing Semiconducting Materials: Synthesis and Applications in Organic Photovoltaics and Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2020, 12, 32209–32232. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Yuan, J.; Zou, Y.; He, D.; Peng, H.; Li, Y.; Zhang, Z. An asymmetric small molecule based on thieno[2,3-f]benzofuran for efficient organic solar cells. Org. Electron. 2016, 35, 87–94. [Google Scholar] [CrossRef]
- Tsuji, H.; Nakamura, E. Design and Functions of Semiconducting Fused Polycyclic Furans for Optoelectronic Applications. Acc. Chem. Res. 2017, 50, 396–406. [Google Scholar] [CrossRef]
- Peng, H.; Luan, X.; Qiu, L.; Li, H.; Liu, Y.; Zou, Y. New naphtho[1,2-b:5,6-b′]difuran based two-dimensional conjugated small molecules for photovoltaic application. Opt. Mater. 2017, 72, 147–155. [Google Scholar] [CrossRef]
- Cao, H.; Rupar, P.A. Recent Advances in Conjugated Furans. Chem. Eur. J. 2017, 23, 14670–14675. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L. Recent Advances of Furan and Its Derivatives Based Semiconductor Materials for Organic Photovoltaics. Small Methods 2021, 5, e2100493. [Google Scholar] [CrossRef]
- Kowada, T.; Ohe, K. Synthesis and Characterization of Highly Fluorescent and Thermally Stable π-Conjugates involving Spiro[fluorene-9,4′-[4H]indeno[1,2-b]furan]. Bull. Korean Chem. Soc. 2010, 31, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Kowada, T.; Kuwabara, T.; Ohe, K. Synthesis, structures, and optical properties of heteroarene-fused dispiro compounds. J. Org. Chem. 2010, 75, 906–913. [Google Scholar] [CrossRef]
- Stobe, C.; Seto, R.; Schneider, A.; Lützen, A. Synthesis, Chiral Resolution, and Absolute Configuration of C2-Symmetric, Chiral 9,9′-Spirobifluorenes. Eur. J. Org. Chem. 2014, 2014, 6513–6518. [Google Scholar] [CrossRef]
- Bhanuchandra, M.; Murakami, K.; Vasu, D.; Yorimitsu, H.; Osuka, A. Transition-Metal-Free Synthesis of Carbazoles and Indoles by an SNAr-Based “Aromatic Metamorphosis” of Thiaarenes. Angew. Chem. Int. Ed. 2015, 54, 10234–10238. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.Q.; Dang, T.T.; Villinger, A.; Sung, T.V.; Langer, P. Efficient synthesis of thieno[3,2-b:4,5-b′]diindoles and benzothieno[3,2-b]indoles by Pd-catalyzed site-selective C-C and C-N coupling reactions. Org. Biomol. Chem. 2012, 10, 9041–9044. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.G.; Saejueng, P.; Murphy, J.M.; Venkataraman, D. Synthesis of 2-Arylbenzo[b]furans via Copper(I)-Catalyzed Coupling of o-Iodophenols and Aryl Acetylenes. Org. Lett. 2002, 4, 4727–4729. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, D.; Chen, Y.H.; Lei, A.; Knochel, P. Preparation of Polyfunctional Biaryl Derivatives by Cyclolanthanation of 2-Bromobiaryls and Heterocyclic Analogues Using nBu2LaCl4·LiCl. Angew. Chem. Int. Ed. 2019, 58, 15631–15635. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Rev. E.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Crystallogr. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]









| λabs (nm) a | λem (nm) b | Φ c | |
|---|---|---|---|
| rac-1 | 337 | 369 (320) | 13 |
| rac-2 | 332, 355 (sh) | 480, 519 (330) | <1 |
| rac-3 | 335, 353 (sh) | 402 (330) | 16 |
| rac-SSd | 340 | 368 (330) | 6 |
| rac-SS(O)2d | 336, 356 (sh) | 459 (380) | 1 |
| rac-SNe | 334, 350 (sh) | 414 (330) | 2 |
| gabs (×10−3) a | glum (×10−3) b | |[α]D25| c | |
|---|---|---|---|
| (+)-1 | +1.2 (341 nm) | +0.90 (381 nm) | 154 (c 0.298) |
| (+)-2 | +1.4 (378 nm) | +2.8 (517 nm) | 151 (c 0.150) |
| (+)-3 | +0.31 (351 nm) | +0.97 (394 nm) | 175 (c 0.094) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, K.; Takase, K.; Noguchi, K. Furan-Containing Chiral Spiro-Fused Polycyclic Aromatic Compounds: Synthesis and Photophysical Properties. Molecules 2022, 27, 5103. https://doi.org/10.3390/molecules27165103
Nakano K, Takase K, Noguchi K. Furan-Containing Chiral Spiro-Fused Polycyclic Aromatic Compounds: Synthesis and Photophysical Properties. Molecules. 2022; 27(16):5103. https://doi.org/10.3390/molecules27165103
Chicago/Turabian StyleNakano, Koji, Ko Takase, and Keiichi Noguchi. 2022. "Furan-Containing Chiral Spiro-Fused Polycyclic Aromatic Compounds: Synthesis and Photophysical Properties" Molecules 27, no. 16: 5103. https://doi.org/10.3390/molecules27165103