Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill
Abstract
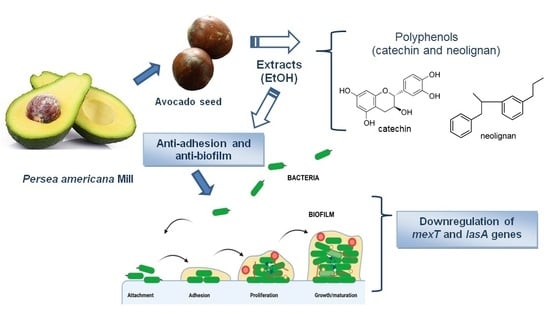
:1. Introduction
2. Results
2.1. Plant Samples Preparation
2.2. Antibacterial Activity and Cytotoxicity of P. americana Seed Ethanol Extracts
2.3. Ethanol Extracts from P. americana Seeds Inhibit Bacterial Adhesion to A549 Epithelial Lung Cells
2.4. Ethanol Extracts from P. americana Seeds Inhibits P. aeruginosa Biofilms
2.5. Ethanol Extracts from P. americana Seeds Affect P. aeruginosa Membrane Integrity
2.6. Effect of Ethanol Extracts from P. americana Seeds on Virulence and Adhesion-Related Genes of P. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Plant Samples Preparation
4.3. Cell Lines, Bacterial Strains, and Culture Conditions
4.4. Cytotoxicity Assessment
4.5. Antimicrobial Assessment
4.5.1. Antimicrobial Screening
Microdilution Method
Bacterial Adherence Assay
4.5.2. Biofilm Formation Assay
4.5.3. Measurement of Release of Nucleic Acids
4.6. Real Time PCR and Gel Electrophoresis Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ismahene, Y. Infectious Diseases, Trade, and Economic Growth: A Panel Analysis of Developed and Developing Countries. J. Knowl. Econ. 2021, 13, 2547–2583. [Google Scholar] [CrossRef]
- Shang, Y.; Li, H.; Zhang, R. Effects of Pandemic Outbreak on Economies: Evidence from Business History Context. Front. Public Health 2021, 9, 632043. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, S.; Robben, C.; Alter, T.; Rossmanith, P.; Mester, P. How to Evaluate Non-Growing Cells—Current Strategies for Determining Antimicrobial Resistance of VBNC Bacteria. Antibiotics 2021, 10, 115. [Google Scholar] [CrossRef]
- Liu, H.; Howell, A.B.; Zhang, D.J.; Khoo, C. A randomized, double-blind, placebo-controlled pilot study to assess bacterial anti-adhesive activity in human urine following consumption of a cranberry supplement. Food Funct. 2019, 10, 7645–7652. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Anti-biofilm activity of grapefruit seed extract against Staphylococcus aureus and Escherichia coli. J. Microbiol. Biotechnol. 2019, 29, 1177–1183. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Klemm, P.; Vejborg, R.; Hancock, V. Prevention of bacterial adhesion. Appl. Microbiol. Biotechnol. 2010, 88, 451–459. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilms and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [Green Version]
- Awololaa, G.V.; Cheniab, H.; Baijnathb, H.; Koorbanallya, N.A. Anti-adhesion potential of non-polar compounds and extracts from Ficus natalensis. Rev. Bras. Farmacogn. 2017, 27, 599–602. [Google Scholar] [CrossRef]
- Ćirić, A.D.; Petrović, J.D.; Glamočlija, J.M.; Smiljković, M.S.; Nikolić, M.M.; Stojković, D.S.; Soković, M.D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- Sánchez, E.; Morales, C.R.; Castillo, S.; Leos-Rivas, C.; García-Becerra, L.; Martínez, D.M.O. Antibacterial and Antibiofilm Activity of Methanolic Plant Extracts against Nosocomial Microorganisms. Evid.-Based Complementary Altern. Med. 2016, 2016, 1572697. [Google Scholar] [CrossRef] [Green Version]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.S.; Murugesan, R. Pseudomonas aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Salem, M.Z.; Mervat, E.H.; Ali, H.M.; Abdel-Megeed, A.; El-Settawy, A.A.; Böhm, M.; Mansour, M.M.; Salem, A.Z. Plants-derived bioactives: Novel utilization as antimicrobial, antioxidant and phytoreducing agents for the biosynthesis of metallic nanoparticles. Microb. Pathog. 2021, 158, 105107. [Google Scholar] [CrossRef]
- Egbuonu, A.C.; Opara, I.C.; Onyeabo, C.; Uchenna, N.O. Proximate, functional, antinutrient and antimicrobial properties of avocado pear (Persea americana) Seeds. J. Nutr. Health. Food Eng. 2018, 8, 00260. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Ruiz-Nicolás, R.; Cornejo-Garrido, J.; Tapia, A.; Yépez-Mulia, L. Antiprotozoal and antimycobacterial activities of Persea americana seeds. BMC Complementary Altern. Med. 2013, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Biyik, H.H.; Onur, M.; Torun, B.; Coban, E.P. Antibacterial and anticandidal effects of the leaf extracts of Persea maericana Mill. Ann. Phytomed. 2018, 7, 88–93. [Google Scholar] [CrossRef]
- Molina, S.C.; Chil, I.; Escalona, J.C.; Picanco, R.N.; González, A.F.; García, J.; Cos, P.; Llauradó, G.M.; Morris, H.J. Bioinsecticide potential of ethanol extracts from Persea americana (Lauraceae) seeds on Aedes aegypti mosquitoes. Acta Biol. Colomb. 2022. Accepted. Available online: https://revistas.unal.edu.co/index.php/actabiol/authorDashboard/submission/96277 (accessed on 1 July 2022).
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Mountcastle, S.E.; Cox, S.C.; Sammons, R.L.; Jabbari, S.; Shelton, R.M.; Kuehne, S.A. A review of co-culture models to study the oral microenvironment and disease. J. Oral Microbiol. 2020, 12, 1773122. [Google Scholar] [CrossRef]
- Dosunmu, E.; Chaudhari, A.A.; Singh, S.R.; Dennis, V.A.; Pillai, S.R. Silver-coated carbon nanotubes downregulate the expression of Pseudomonas aeruginosa virulence genes: A potential mechanism for their antimicrobial effect. Int. J. Nanomed. 2015, 10, 5025. [Google Scholar] [CrossRef] [Green Version]
- Janecki, A.; Kolodziej, H. Anti-Adhesive Activities of Flavan-3-ols and Proanthocyanidins in the Interaction of Group A-Streptococci and Human Epithelial Cells. Molecules 2010, 15, 7139–7152. [Google Scholar] [CrossRef] [Green Version]
- Molina, S.C.; Martins, P.; Reyes, B.; Queiroz, M.M.; Escalona, J.C.; García, J.; Guisado, F. Effects of Persea americana Mill. seed extracts on the postembryonic development of Musca domestica (Diptera: Muscoide). J. Pharm. Pharmacogn. Res. 2018, 6, 96–107. [Google Scholar]
- Soledad, C.P.T.; Paola, H.C.; Enrique, O.V.C.; Israel, R.L.I.; GuadalupeVirginia, N.M.; Raúl, Á.S. Avocado seeds (Persea americana cv. Criollo sp.): Lipophilic compounds profile and biological activities. Saudi J. Biol. Sci. 2021, 28, 3384–3390. [Google Scholar] [CrossRef]
- Raymond Chia, T.W.; Dykes, G.A. Antimicrobial activity of crude epicarp and seed extracts from mature avocado fruit (Persea americana) of three cultivars. Pharm. Biol. 2010, 48, 753–756. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [Green Version]
- Kurnia, D.; Ramadhanty, Z.F.; Ardani, A.M.; Zainuddin, A.; Dharsono, H.D.A.; Satari, M.H. Bio-mechanism of catechin as pheromone signal inhibitor: Prediction of antibacterial agent action mode by in vitro and in silico study. Molecules 2021, 26, 6381. [Google Scholar] [CrossRef]
- Bhaskar, A.; Sekhar, S.; Javaraiah, R. Antibiofilm and anti-inflammatory potential of lignans with collegial effect: Secoisolariciresinol diglucoside and sesamin as antimicrobial sources. J. Appl. Biol. Biotechnol. 2020, 8, 45–51. [Google Scholar] [CrossRef]
- Keawchai, K.; Chumkaew, P.; Permpoonpattana, P.; Srisawat, T. Synergistic effect of ampicillin and dihydrobenzofuran neolignans (myticaganal C) identified from the seeds of Myristica fragrans Houtt. against Escherichia coli. J. Adv. Pharm. Technol. Res. 2021, 12, 79. [Google Scholar] [CrossRef]
- Van der Schalk, T.E.; Coppens, J.; Timbermont, L.; Turlej-Rogacka, A.; Van Heirstraeten, L.; Berkell, M.; Yu, L.; Lammens, C.; Xavier, B.B.; Matheeussen, V.; et al. Evaluation of GeneXpert PA assay compared to genomic and (semi-)quantitative culture methods for direct detection of Pseudomonas aeruginosa in endotracheal aspirates. Antimicrob. Resist. Infect. Control 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Mahavy, C.E.; Duez, P.; ElJaziri, M.; Rasamiravaka, T. African Plant-Based Natural Products with Antivirulence Activities to the Rescue of Antibiotics. Antibiotics 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.L.; van Delden, C.; Perron, K.; Kohler, T. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol. Lett. 2006, 254, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.J.; Oliveira, M.R.; Rocha, R.R.; Laurindo, M.V.; Rocha, S.L.; Laurindo de Moraes, F.L. Pseudomonas aeruginosa: Virulence Factors and Antibiotic Resistance Genes. Braz. Arch. Biol. Technol. 2019, 62, e19180503. [Google Scholar] [CrossRef]
- Mapipa, Q.; Digban, T.O.; Nnolim, N.E.; Nwodo, U.U. Antibiogram profile and virulence signatures of Pseudomonas aeruginosa isolates recovered from selected agrestic hospital effluents. Sci. Rep. 2021, 11, 11800. [Google Scholar] [CrossRef]
- Khayat, M.; Ibrahim, T.; Khayyat, A.N.; Alharbi, M.; Shaldam, M.A.; Mohammad, K.A.; Khafagy, E.; El-damasy, D.A.; Hegazy, W.A.; Abbas, H.A. Sodium Citrate Alleviates Virulence in Pseudomonas aeruginosa. Microorganisms 2022, 10, 1046. [Google Scholar] [CrossRef]
- Tian, Z.X.; Mac Aogain, M.; O’Connor, H.F.; Fargier, E.; Mooij, M.J.; Adams, C.M.; Wang, Y.P.; O’Gara, F. MexT modulates virulence determinants in Pseudomonas aeruginosa independent of the MexEF-OprN efflux pump. Microb. Pathog. 2009, 47, 237–241. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Tran, M.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Muñoz-Cazares, N.; García-Contreras, R.; Pérez-López, M.; Castillo-Juárez, I. Phenolic compounds with anti-virulence properties. In Phenolic Compounds-Biological Activity; Soto-Hernández, M., Palma Tenango, M., García-Mateo, M.R., Eds.; IntechOpen: London, UK, 2017; pp. 139–167. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane disruption properties of essential oils—A double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Berenguer-Rivas, C.A.; Escalona-Arranz, J.C.; Llauradó-Maury, G.; Van der Auwera, A.; Piazza, S.; Méndez-Rodríguez, D.; Foubert, K.; Cos, P.; Pieters, L. Anti-inflammatory effect of Adelia ricinella L. aerial parts. J. Pharm. Pharmacol. 2021, 73, 553–559. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Casas-Forero, N.; Pastene-Navarrete, E.; Lima Silva, F.; Alarcón-Enos, J. Fractionation and hydrolyzation of avocado peel extract: Improvement of antibacterial activity. Antibiotics 2020, 10, 23. [Google Scholar] [CrossRef]
- Cools, F.; Torfs, E.; Vanhoutte, B.; de Macedo, M.B.; Bonofiglio, L.; Mollerach, M.; Cos, P. Streptococcus pneumoniae galU gene mutation has a direct effect on biofilm growth, adherence and phagocytosis in vitro and pathogenicity in vivo. Pathog. Dis. 2018, 76, fty069. [Google Scholar] [CrossRef] [Green Version]
- Toté, K.; Berghe, D.V.; Deschacht, M.; De Wit, K.; Maes, L.; Cos, P. Inhibitory efficacy of various antibiotics on matrix and viable mass of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Int. J. Antimicrob. Agents 2009, 33, 525–531. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Cao, H.; Lai, Y.; Bougouffa, S.; Xu, Z.; Yan, A. Comparative genome and transcriptome analysis reveals distinctive surface characteristics and unique physiological potentials of Pseudomonas aeruginosa ATCC 27853. BMC Genom. 2017, 18, 459. [Google Scholar] [CrossRef] [Green Version]





| Bacteria | IC50 | |||
|---|---|---|---|---|
| SE (µg/mL) | MaE (µg/mL) | Doxycycline (µM) | Ciprofloxacin (µM) | |
| E. coli ATCC 8739 | ˃1024 | ˃1024 | 0.50 ± 0.28 | - |
| P. aeruginosa ATCC 9027 | 87.0 ± 4.4 * | 187.4 ± 9.4 * | - | 1.05 ± 0.50 |
| S. aureus ATCC 6538 | 144.2 ± 5.7 * | 159.2 ± 7.9 | 0.04 ± 0.95 | - |
| S. pneumoniae NTCC 7466 | 227.6 ± 11.4 | 532.2 ± 26.6 | 3.56 ± 0.84 | - |
| Cell Lines | CC50 | ||
|---|---|---|---|
| SE (µg/mL) | MaE (µg/mL) | Tamoxifen (µM) | |
| MRC-5 | 636.9 ± 31.8 | 786.3 ± 39.3 | 8.7 ± 0.4 |
| A549 | 770.0 ± 38.5 | 774.3 ± 38.7 | 6.1 ± 0.3 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| mexT | ACCTCATGGGTTGTGACTGTATCC | TAGGATCACTGACAGGCATAGCCA |
| oprD | TTTCCGCAGGTAGCACTCAGT | CTTCGCTTCGGCCTGATC |
| lasA | TTCTGTGATCGATTCGGCTCGGTT | ACCCGGGAAGACAACTATCAGCTT |
| lecA | CACCATTGTGTTTCCTGGCGTTCA | AGAAGGCAACGTCGACTCGTTGAT |
| lecB | AGACAGCGTAACAATCGAACGAGC | AGGACGCATCGTTCAGCCAATCTA |
| rpoS | CGGCGAGTTGGTCATCATCAAACA | ATCGATTGCCCTACCTTGACCTGT |
| prtR | TCCCTGCACCCATGTGAAATCTCT | ATCGGCAATCTACAGACCGATGGA |
| creD | CGCCATCGCCCTACTCAT | GGCGATCGCGGATCAG |
| ampG | GGCCTGACCTATGCGTTGTT | CGACGGGTTGACGTGGAT |
| ampP | ACCGCCGCCTGGTCAT | CAGGCCGATGGGAATGC |
| creB | GCATATCCTGATCGTCGAAGATG | GGCCTGCAGGGCGTAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina Bertrán, S.d.C.; Monzote, L.; Cappoen, D.; Escalona Arranz, J.C.; Gordillo Pérez, M.J.; Rodríguez-Ferreiro, A.O.; Chill Nuñez, I.; Novo, C.P.; Méndez, D.; Cos, P.; et al. Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill. Molecules 2022, 27, 5009. https://doi.org/10.3390/molecules27155009
Molina Bertrán SdC, Monzote L, Cappoen D, Escalona Arranz JC, Gordillo Pérez MJ, Rodríguez-Ferreiro AO, Chill Nuñez I, Novo CP, Méndez D, Cos P, et al. Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill. Molecules. 2022; 27(15):5009. https://doi.org/10.3390/molecules27155009
Chicago/Turabian StyleMolina Bertrán, Silvia del Carmen, Lianet Monzote, Davie Cappoen, Julio Cesar Escalona Arranz, Mario Juan Gordillo Pérez, Annarli O. Rodríguez-Ferreiro, Idelsy Chill Nuñez, Claudina Pérez Novo, Daniel Méndez, Paul Cos, and et al. 2022. "Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill" Molecules 27, no. 15: 5009. https://doi.org/10.3390/molecules27155009