The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems
Abstract
:1. Introduction
2. Metal–Organic Structures: Synthesis Strategies
2.1. Methods of Synthesis
2.1.1. Reticular Synthesis
2.1.2. Hydrothermal–Solvothermal Synthesis
2.1.3. Diffusion Synthesis
2.1.4. Electrochemical Synthesis
2.1.5. Microwave-Assisted Synthesis
2.1.6. Mechanochemical Synthesis
2.1.7. Sonochemical Synthesis
3. Metal–Organic Frameworks
3.1. Properties
3.2. Applications
3.2.1. Adsorption
3.2.2. Catalysis
3.2.3. Drug Delivery
3.2.4. Sensors
3.2.5. Hydrogen Storage
3.2.6. Environmental Applications
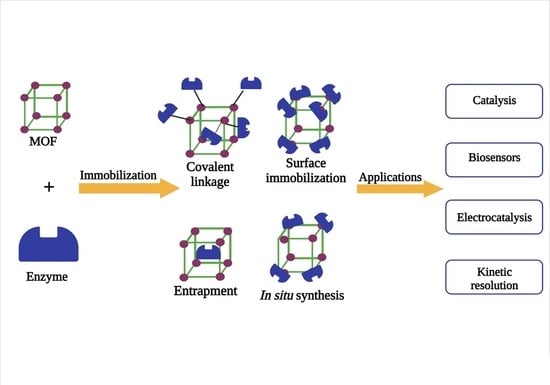
4. Enzyme Immobilization with Metal–Organic Frameworks (MOFs)
4.1. In Situ Synthesis
4.2. Covalent Bonding
4.3. Surface Immobilization
4.4. Entrapment
5. Future Trends
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rocha, T.G.; Pedro, P.H.; de Souza, M.C.M.; Monteiro, R.R.C.; dos Santos, J.C.S. Lipase Cocktail for Optimized Biodiesel Production of Free Fatty Acids from Residual Chicken Oil. Catal. Lett. 2021, 151, 1155–1166. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; da Rocha, T.N.; dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Biotechnological Relevance of the Lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; Dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases a and b from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcante, F.T.T.d.A.; Falcão, I.R.; Souza, d.S.J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Moreira, K.S.; Moura, L.S.; Monteiro, R.R.C.; de Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; de Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya Sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Valério, R.B.R.; Cavalcante, A.L.G.; Mota, G.F.; de Sousa, I.G.; da Silva Souza, J.E.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Aguiar Falcão, I.R.; Neto, F.S.; Dos Santos, J.C.S. Understanding the Biocatalytic Potential of Lipase from Rhizopus Chinensis. Biointerface Res. Appl. Chem. 2022, 12, 4230–4260. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase Ultra: A Phospholipase with Great Potential in Biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.E.S.; Monteiro, R.R.C.; Rocha, T.G.; Moreira, K.S.; Cavalcante, F.T.T.; de Sousa Braz, A.K.; de Souza, M.C.M.; dos Santos, J.C.S. Sonohydrolysis Using an Enzymatic Cocktail in the Preparation of Free Fatty Acid. 3 Biotech 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Rocha-Martin, J.; Santos, J.C.S. dos Editorial: Designing Carrier-Free Immobilized Enzymes for Biocatalysis. Front. Bioeng. Biotechnol. 2022, 10, 1–5. [Google Scholar] [CrossRef]
- Melo, A.D.Q.; Silva, F.F.M.; Dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [Green Version]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; Gomes e Silva, N.C.; Souza, J.E.d.S.; Nunes, Y.L.; Sousa dos Santos, J.C. An Overview on the Conversion of Glycerol to Value-Added Industrial Products via Chemical and Biochemical Routes. Biotechnol. Appl. Biochem. 2021, 0, 1–25. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.M.; de Freitas, Í.B.; Soares, N.B.; de Araújo, F.A.M.; Gaieta, E.M.; Dos Santos, J.C.S.; Sobrinho, A.C.N.; Marinho, E.S.; Colares, R.P. Synthesis, Biological Activity, and in Silico Study of Bioesters Derived from Bixin by the Calb Enzyme. Biointerface Res. Appl. Chem. 2022, 12, 5901–5917. [Google Scholar] [CrossRef]
- Serpa, J.d.F.; Matias, G.A.B.; Fechine, P.B.A.; da Costa, V.M.; Freire, R.M.; Denardin, J.C.; Gonçalves, L.R.B.; de Macedo, A.C.; Rocha, M.V.P. New Nanocomposite Made of Cashew Apple Bagasse Lignin and Fe3O4 for Immobilizing of Lipase B from Candida antarctica Aiming at Esterification. J. Chem. Technol. Biotechnol. 2021, 96, 2472–2487. [Google Scholar] [CrossRef]
- Mota, G.F.; de Sousa, I.G.; de Oliveira, A.L.B.; Cavalcante, A.L.G.; da Silva Moreira, K.; Cavalcante, F.T.T.; da Silva Souza, J.E.; de Aguiar Falcão, Í.R.; Guimarães Rocha, T.; Bussons Rodrigues Valério, B.R.R.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Chaves, A.V.; Fechine, P.B.A.; Holanda Alexandre, J.Y.N.; Freire, T.M.; Davi, D.M.B.; Neto, F.S.; de Sousa, I.G.; da Silva Moreira, K.; de Oliveira, A.L.B.; et al. Chemical Modification of Clay Nanocomposites for the Improvement of the Catalytic Properties of Lipase A from Candida antarctica. Process Biochem. 2022, 120, 1–14. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid Lipase Preparations Designed for Industrial Production of Biodiesel. Is It Really an Optimal Solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Moreira, K.d.S.; de Oliveira, A.L.B.; de Moura, S.L.J.; de Sousa, I.G.; Cavalcante, L.G.A.; Neto, F.S.; Valério, R.B.R.; Chaves, V.A.; de Sousa, T.F.; Cruz, M.V.D.; et al. Taguchi Design-Assisted Co-Immobilization of Lipase A and B from Candida antarctica onto Chitosan: Characterization, Kinetic Resolution Application, and Docking Studies. Chem. Eng. Res. Des. 2022, 177, 223–244. [Google Scholar] [CrossRef]
- Souza, J.E.d.S.; de Oliveira, G.P.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.d.S.; de Oliveira, A.L.B.; de Souza, M.C.M.; dos Santos, J.C.S. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89–113. [Google Scholar] [CrossRef]
- Gao, Y.; Shah, K.; Kwok, I.; Wang, M.; Rome, L.H.; Mahendra, S. Immobilized Fungal Enzymes: Innovations and Potential Applications in Biodegradation and Biosynthesis. Biotechnol. Adv. 2022, 57, 107936. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F. Cell–Enzyme Tandem Systems for Sustainable Chemistry. Curr. Opin. Green Sustain. Chem. 2022, 34, 100600. [Google Scholar] [CrossRef]
- Green, J.J.; Elisseeff, J.H. Mimicking Biological Functionality with Polymers for Biomedical Applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Richardson, J. In Organic Solvents. Heteroat. Analog. Aldehydes Ketones 2004, 409, 1. [Google Scholar] [CrossRef]
- Lin, C.; Xu, K.; Zheng, R.; Zheng, Y. Immobilization of Amidase into a Magnetic Hierarchically Porous Metal-Organic Framework for Efficient Biocatalysis. Chem. Commun. 2019, 55, 5697–5700. [Google Scholar] [CrossRef]
- Cui, J.D.; Jia, S.R. Optimization Protocols and Improved Strategies of Cross-Linked Enzyme Aggregates Technology: Current Development and Future Challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Guo, X.; Zheng, Q.; Peng, J.; Tang, H.; Yao, S. Carbon Nanotubes Labeled with Aptamer and Horseradish Peroxidase as a Probe for Highly Sensitive Protein Biosensing by Postelectropolymerization of Insoluble Precipitates on Electrodes. Anal. Chem. 2015, 87, 7610–7617. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Doderick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Insight Review Articles Expansion Phase. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Jung, D. Biocatalysis with Enzymes Immobilized on Mesoporous Hosts: The Status Quo and Future Trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Feng, W.; Ji, P. Enzymes Immobilized on Carbon Nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme Immobilisation in Biocatalysis: Why, What and How. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Kondrat, S.; Krauss, U.; von Lieres, E. Enzyme Co-Localisation: Mechanisms and Benefits. Curr. Res. Chem. Biol. 2022, 2, 100031. [Google Scholar] [CrossRef]
- Patti, A.; Sanfilippo, C. Stereoselective Promiscuous Reactions Catalyzed by Lipases. Int. J. Mol. Sci. 2022, 23, 2675. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, C.M.; Pelletier, J.N. Expanding the Organic Toolbox: A Guide to Integrating Biocatalysis in Synthesis. Chem. Soc. Rev. 2012, 41, 1585–1605. [Google Scholar] [CrossRef]
- Muñoz Solano, D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial Biotransformations in the Synthesis of Building Blocks Leading to Enantiopure Drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R. Biocatalysis-Key to Sustainable Industrial Chemistry. Curr. Opin. Biotechnol. 2010, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hartmann, M. Progress in Enzyme Immobilization in Ordered Mesoporous Materials and Related Applications. Chem. Soc. Rev. 2013, 42, 3894–3912. [Google Scholar] [CrossRef] [PubMed]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P.M. Immobilised Enzymes in Biorenewables Production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [Green Version]
- Cantone, S.; Hanefeld, U.; Basso, A. Biocatalysis in Non-Conventional Media—Ionic Liquids, Supercritical Fluids and the Gas Phase. Green Chem. 2007, 9, 954–997. [Google Scholar] [CrossRef]
- Gao, C.; Zhu, H.; Chen, J.; Qiu, H. Facile Synthesis of Enzyme Functional Metal-Organic Framework for Colorimetric Detecting H2O2 and Ascorbic Acid. Chin. Chem. Lett. 2017, 28, 1006–1012. [Google Scholar] [CrossRef]
- Xu, C.P.; Yun, J.W. Influence of Aeration on the Production and the Quality of the Exopolysaccharides from Paecilomyces Tenuipes C240 in a Stirred-Tank Fermenter. Enzym. Microb. Technol. 2004, 35, 33–39. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.C.; Silva, F.F.M.; Lemos, T.L.G.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Modulation of Lecitase Properties via Immobilization on Differently Activated Immobead-350: Stabilization and Inversion of Enantiospecificity. Process Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; Dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.H. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.M.; Monteiro, R.R.C.; Neto, D.M.A.; da Silva, F.F.M.; de Paula, R.C.M.; de Lemos, T.L.G.; Fechine, P.B.A.; Correa, M.A.; Bohn, F.; Gonçalves, L.R.B.; et al. A New Heterofunctional Support for Enzyme Immobilization: PEI Functionalized Fe3O4 MNPs Activated with Divinyl Sulfone. Application in the Immobilization of Lipase from Thermomyces Lanuginosus. Enzym. Microb. Technol. 2020, 138, 109560. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of Lipases on Heterofunctional Octyl-Glyoxyl Agarose Supports Improved Stability and Prevention of the Enzyme Desorption. Methods Enzymol. 2016, 571, 73–85. [Google Scholar]
- Husain, Q. Nanomaterials as Novel Supports for the Immobilization of Amylolytic Enzymes and Their Applications: A Review. Biocatalysis 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas, L.E.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal-Organic Frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Mishra, H.; Srivastava, A.; Kayastha, A.M. Covalent Immobilization of Β-Amylase onto Functionalized Molybdenum Sulfide Nanosheets, Its Kinetics and Stability Studies: A Gateway to Boost Enzyme Application. Chem. Eng. J. 2017, 328, 215–227. [Google Scholar] [CrossRef]
- Ding, C.; Sun, H.; Ren, J.; Qu, X. Immobilization of Enzyme on Chiral Polyelectrolyte Surface. Anal. Chim. Acta 2017, 952, 88–95. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-Point Enzyme Immobilization, Surface Chemistry, and Novel Platforms: A Paradigm Shift in Biocatalyst Design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Sousa, E.Y.A.; de França Serpa, J.; Oliveira, R.C.; Dos Santos, J.C.S. Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; dos Santos, J.C.S.; Mammarella, E.J. Operational and Thermal Stability Analysis of Thermomyces Lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl. Biochem. Biotechnol. 2018, 184, 182–196. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, A.M.; Cleiton Sousa dos Santos, J.; de Souza, M.C.M.; de Oliveira, M.M.; Colares, R.P.; de Lemos, T.L.G.; Braz-Filho, R. The Use of New Hydrogel Microcapsules in Coconut Juice as Biocatalyst System for the Reaction of Quinine. Ind. Crops Prod. 2020, 145, 111890. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; De Sant’Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Stabilizing Hyperactivated Lecitase Structures through Physical Treatment with Ionic Polymers. Process Biochem. 2014, 49, 1511–1515. [Google Scholar] [CrossRef]
- de Souza, T.C.; de Sousa Fonseca, T.; de Sousa Silva, J.; Lima, P.J.M.; Neto, C.A.C.G.; Monteiro, R.R.C.; Rocha, M.V.P.; de Mattos, M.C.; dos Santos, J.C.S.; Gonçalves, L.R.B. Modulation of Lipase B from Candida antarctica Properties via Covalent Immobilization on Eco-Friendly Support for Enzymatic Kinetic Resolution of Rac-Indanyl Acetate. Bioprocess Biosyst. Eng. 2020, 43, 2253–2268. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.L.B.; Cavalcante, F.T.T.; Moreira, K.S.; Monteiro, R.R.C.; Rocha, T.G.; Souza, J.E.S.; da Fonseca, A.M.; Lopes, A.A.S.; Guimarães, A.P.; de Lima, R.K.C.; et al. Lipases Immobilized onto Nanomaterials as Biocatalysts in Biodiesel Production: Scientific Context, Challenges, and Opportunities. Rev. Virtual Quim. 2021, 13, 875–891. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Cavalcante, C.G.; Colares, R.P.; Ferreira, D.A.; da Silva, F.F.M.; de Sousa, E.Y.A.; da Silva Souza, J.E.; de Castro Monteiro, R.R.; de Oliveira, A.L.B.; dos Santos, J.C.S.; et al. Preparation, Characterization, and Enantioselectivity of Polyacrylate Microcapsules Entrapping Ananas Comosus Extract. Rev. Virtual Quim. 2021, 13, 1319–1329. [Google Scholar] [CrossRef]
- Carneiro, E.; Bastos, A.; De Oliveira, U.; De Matos, L.; Adriano, W.; Monteiro, R.; Dos Santos, J.; Gonçalves, L. Improving the Catalytic Features of the Lipase from Rhizomucor Miehei Immobilized on Chitosan-Based Hybrid Matrices by Altering the Chemical Activation Conditions. Quim. Nova 2020, 43, 1234–1239. [Google Scholar] [CrossRef]
- Rios, N.S.; Morais, E.G.; dos Santos Galvão, W.; Andrade Neto, D.M.; dos Santos, J.C.S.; Bohn, F.; Correa, M.A.; Fechine, P.B.A.; Fernandez-Lafuente, R.; Gonçalves, L.R.B. Further Stabilization of Lipase from Pseudomonas Fluorescens Immobilized on Octyl Coated Nanoparticles via Chemical Modification with Bifunctional Agents. Int. J. Biol. Macromol. 2019, 141, 313–324. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Kambale, S.; Jadhav, S.; Nadar, S.; Ladole, M. A Tri-Enzyme Magnetic Nanobiocatalyst with One Pot Starch Hydrolytic Activity. Chem. Eng. J. 2017, 325, 80–90. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Yue, S.; Zhao, Y.; Li, L.; Liu, R.; Lin, T. Magnetic Mesoporous Enzyme-Silica Composites with High Activity and Enhanced Stability. J. Chem. Technol. Biotechnol. 2016, 91, 1905–1913. [Google Scholar] [CrossRef]
- Cui, J.; Feng, Y.; Jia, S. Silica Encapsulated Catalase@metal-Organic Framework Composite: A Highly Stable and Recyclable Biocatalyst. Chem. Eng. J. 2018, 351, 506–514. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan Beads from Microbial and Animal Sources as Enzyme Supports for Wine Application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Lee, K.Y.; Yuk, S.H. Polymeric Protein Delivery Systems. Prog. Polym. Sci. 2007, 32, 669–697. [Google Scholar] [CrossRef]
- Lee, E.S.; Kwon, M.J.; Lee, H.; Kim, J.J. Stabilization of Protein Encapsulated in Poly(Lactide-Co-Glycolide) Microspheres by Novel Viscous S/W/O/W Method. Int. J. Pharm. 2007, 331, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Vakhshiteh, F.; Barkhi, M.; Baharifar, H.; Poor-Akbar, E.; Zari, N.; Stamatis, H.; Bordbar, A.K. Immobilization of Cellulase Enzyme onto Magnetic Nanoparticles: Applications and Recent Advances. Mol. Catal. 2017, 442, 66–73. [Google Scholar] [CrossRef]
- Hong, S.G.; Kim, B.C.; Na, H.B.; Lee, J.; Youn, J.; Chung, S.W.; Lee, C.W.; Lee, B.; Kim, H.S.; Hsiao, E.; et al. Single Enzyme Nanoparticles Armored by a Thin Silicate Network: Single Enzyme Caged Nanoparticles. Chem. Eng. J. 2017, 322, 510–515. [Google Scholar] [CrossRef]
- Fried, D.I.; Brieler, F.J.; Fröba, M. Designing Inorganic Porous Materials for Enzyme Adsorption and Applications in Biocatalysis. ChemCatChem 2013, 5, 862–884. [Google Scholar] [CrossRef]
- Kato, K.; Kawachi, Y.; Nakamura, H. Silica-Enzyme-Ionic Liquid Composites for Improved Enzymatic Activity. J. Asian Ceram. Soc. 2014, 2, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Iyer, P.V.; Ananthanarayan, L. Enzyme Stability and Stabilization-Aqueous and Non-Aqueous Environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Magner, E. Proteins in Mesoporous Silicates. Angew. Chem. Int. Ed. 2008, 47, 8582–8594. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Dos Santos, J.C.S.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, K.d.S.; de Oliveira, A.L.B.; Júnior, L.S.d.M.; Monteiro, R.R.C.; da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.U.D.; Denardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase From Rhizomucor Miehei Immobilized on Magnetic Nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) Optimized Production by the Taguchi Method. Front. Bioeng. Biotechnol. 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of Lipase a from Candida antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Zhong, L.; Jia, S.; Cui, J. Acid-Resistant Enzyme@MOF Nanocomposites with Mesoporous Silica Shells for Enzymatic Applications in Acidic Environments. J. Biotechnol. 2019, 306, 54–61. [Google Scholar] [CrossRef]
- Sun, Q.; Pan, Y.; Wang, X.; Li, H.; Farmakes, J.; Aguila, B.; Yang, Z.; Ma, S. Mapping out the Degree of Freedom of Hosted Enzymes in Confined Spatial Environments. Chem 2019, 5, 3184–3195. [Google Scholar] [CrossRef]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and Physical Chitosan Modification for Designing Enzymatic Industrial Biocatalysts: How to Choose the Best Strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef]
- Srbová, J.; Slováková, M.; Křípalová, Z.; Žárská, M.; Špačková, M.; Stránská, D.; Bílková, Z. Covalent Biofunctionalization of Chitosan Nanofibers with Trypsin for High Enzyme Stability. React. Funct. Polym. 2016, 104, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.W.; Jeon, B.H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.B.; Cao, R. Metal–Organic Frameworks and Porous Organic Polymers for Sustainable Fixation of Carbon Dioxide into Cyclic Carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Marsh, C.; Shearer, G.C.; Knight, B.T.; Paul-Taylor, J.; Burrows, A.D. Supramolecular Aspects of Biomolecule Interactions in Metal–Organic Frameworks. Coord. Chem. Rev. 2021, 439, 213928. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, B. A Point-of-Care Diagnostics Logic Detector Based on Glucose Oxidase Immobilized Lanthanide Functionalized Metal-Organic Frameworks. Nanoscale 2019, 11, 22946–22953. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, J.; Xue, J.; Liang, K. Metal–Organic Frameworks as a Versatile Materials Platform for Unlocking New Potentials in Biocatalysis. Small 2021, 17, 1–21. [Google Scholar] [CrossRef]
- Birhanlı, E.; Noma, S.A.A.; Boran, F.; Ulu, A.; Yeşilada, Ö.; Ateş, B. Design of Laccase–Metal–Organic Framework Hybrid Constructs for Biocatalytic Removal of Textile Dyes. Chemosphere 2022, 292, 133382. [Google Scholar] [CrossRef] [PubMed]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic Applications of Enzymes Encapsulated in Metal–Organic Frameworks. Coord. Chem. Rev. 2019, 381, 151–160. [Google Scholar] [CrossRef]
- Pérez Gascón, V.; Sánchez-Sánchez, M. Environmentally friendly enzyme immobilization on MOF materials. In Immobilization of Enzymes and Cells: Methods and Protocols; Guisan, J.M., Bolivar, J.M., López-Gallego, F., Rocha-Martín, J., Eds.; Humana: New York, NY, USA, 2020; pp. 271–296. ISBN 978-1-0716-0215-7. [Google Scholar]
- Sun, H.; Li, Y.; Yu, S.; Liu, J. Metal-Organic Frameworks (MOFs) for Biopreservation: From Biomacromolecules, Living Organisms to Biological Devices. Nano Today 2020, 35, 100985. [Google Scholar] [CrossRef]
- Huang, S.; Kou, X.; Shen, J.; Chen, G.; Ouyang, G. “Armor-Plating” Enzymes with Metal–Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2020, 59, 8786–8798. [Google Scholar] [CrossRef]
- Cong, W.J.; Nanda, S.; Li, H.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Metal-Organic Framework-Based Functional Catalytic Materials for Biodiesel Production: A Review. Green Chem. 2021, 23, 2595–2618. [Google Scholar] [CrossRef]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal-Organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications. Front. Bioeng. Biotechnol. 2020, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S. Metal–Organic Frameworks for Biosensing and Bioimaging Applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.L.; Xiong, J.; Zong, M.H.; Lou, W.Y. Metal-Organic Frameworks as Novel Matrices for Efficient Enzyme Immobilization: An Update Review. Coord. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal-Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, Y.; Liu, S.; Wu, D.; Su, Z.; Chen, G.; Liu, J.; Li, G. Recent Advances in Enzyme Immobilization Based on Novel Porous Framework Materials and Its Applications in Biosensing. Coord. Chem. Rev. 2022, 459, 214414. [Google Scholar] [CrossRef]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-Organic Frameworks with Different Dimensionalities: An Ideal Host Platform for Enzyme@MOF Composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.C. Enzyme-MOF (Metal-Organic Framework) Composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination Polymers, Metal-Organic Frameworks and the Need for Terminology Guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef] [Green Version]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Kumar, P.; Vellingiri, K.; Kim, K.H.; Brown, R.J.C.; Manos, M.J. Modern Progress in Metal-Organic Frameworks and Their Composites for Diverse Applications. Microporous Mesoporous Mater. 2017, 253, 251–265. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal-Organic Framework Composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Huskić, I.; Pekov, I.V.; Krivovichev, S.V.; Friščić, T. Minerals with Metal-Organic Framework Structures. Sci. Adv. 2016, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Doherty, C.M.; Buso, D.; Hill, A.J.; Furukawa, S.; Kitagawa, S.; Falcaro, P. Using Functional Nano- and Microparticles for the Preparation of Metal-Organic Framework Composites with Novel Properties. Acc. Chem. Res. 2014, 47, 396–405. [Google Scholar] [CrossRef]
- Coudert, F.X.; Fuchs, A.H. Computational Characterization and Prediction of Metal-Organic Framework Properties. Coord. Chem. Rev. 2016, 307, 211–236. [Google Scholar] [CrossRef] [Green Version]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Thouvenot, C.; Noguès, M.; Marsolier, G.; Louër, D.; Férey, G. Very Large Breathing Effect in the First Nanoporous Chromium(III)-Based Solids: MIL-53 or CrIII(OH)·{O2C-C6H4- CO2}·{HO2C-C6H4 -CO2H}x·H2Oy. J. Am. Chem. Soc. 2002, 124, 13519–13526. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Han, C.; Li, M.; Guo, L. Electrocatalytically Active Cobalt-Based Metal-Organic Framework with Incorporated Macroporous Carbon Composite for Electrochemical Applications. J. Mater. Chem. A 2015, 3, 732–738. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal-Organic Frameworks—Prospective Industrial Applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Azizian, S. Nanoporous Carbon Derived from MOF-5: A Superadsorbent for Copper Ions. ACS Omega 2018, 3, 16954–16959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Ryu, U.J.; Kwon, W.; Choi, K.M. A Multi-Dye Containing MOF for the Ratiometric Detection and Simultaneous Removal of Cr2O72− in the Presence of Interfering Ions. Sens. Actuators B Chem. 2019, 283, 426–433. [Google Scholar] [CrossRef]
- Oveisi, M.; Alinia Asli, M.; Mahmoodi, N.M. Carbon Nanotube Based Metal-Organic Framework Nanocomposites: Synthesis and Their Photocatalytic Activity for Decolorization of Colored Wastewater. Inorg. Chim. Acta 2019, 487, 169–176. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Desmet, G.; Ameloot, R.; De Vos, D.E.; Terryn, H.; Denayer, J.F.M. Electrochemical Synthesis of Thin HKUST-1 Layers on Copper Mesh. Microporous Mesoporous Mater. 2012, 158, 209–213. [Google Scholar] [CrossRef]
- Campagnol, N.; Rezende Souza, E.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent Terbium-Containing Metal–Organic Framework Films: New Approaches for the Electrochemical Synthesis and Application as Detectors for Explosives. Chem. Commun. 2014, 50, 12680–12683. [Google Scholar] [CrossRef] [Green Version]
- Jhung, S.H.; Chang, J.S.; Hwang, J.S.; Park, S.E. Selective Formation of SAPO-5 and SAPO-34 Molecular Sieves with Microwave Irradiation and Hydrothermal Heating. Microporous Mesoporous Mater. 2003, 64, 33–39. [Google Scholar] [CrossRef]
- Jhung, S.H.; Yoon, J.W.; Hwang, J.S.; Cheetham, A.K.; Chang, J.S. Facile Synthesis of Nanoporous Nickel Phosphates without Organic Templates under Microwave Irradiation. Chem. Mater. 2005, 17, 4455–4460. [Google Scholar] [CrossRef]
- Ratera, I.; Veciana, J. Playing with Organic Radicals as Building Blocks for Functional Molecular Materials. Chem. Soc. Rev. 2012, 41, 303–349. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-Free Synthesis of a Microporous Metal-Organic Framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic Strategies, Structure Patterns, and Emerging Properties in the Chemistry of Modular Porous Solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal-Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- MICHL, J. The “Molecular Tinkertoy” approach to materials. In Applications of Organometallic Chemistry in the Preparation and Processing of Advanced Materials; Springer: Dordrecht, The Netherlands, 1995; ISBN 9789401041492. [Google Scholar]
- Jarrah, A.; Farhadi, S. Dawson-Type Polyoxometalate Incorporated into Nanoporous MIL-101(Cr): Preparation, Characterization and Application for Ultrafast Removal of Organic Dyes. Acta Chim. Slov. 2019, 66, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Yu, F.; Xiong, X.; Zhou, L.Y.; Li, J.L.; Liang, J.Y.; Hu, S.Q.; Lu, W.T.; Li, B.; Zhou, H.C. Hierarchical Nickel/Phosphorus/Nitrogen/Carbon Composites Templated by One Metal-Organic Framework as Highly Efficient Supercapacitor Electrode Materials. J. Mater. Chem. A 2019, 7, 2875–2883. [Google Scholar] [CrossRef]
- Gao, T.; Dong, B.X.; Pan, Y.M.; Liu, W.L.; Teng, Y.L. Highly Sensitive and Recyclable Sensing of Fe3+ Ions Based on a Luminescent Anionic [Cd(DMIPA)]2- Framework with Exposed Thioether Group in the Snowflake-like Channels. J. Solid State Chem. 2019, 270, 493–499. [Google Scholar] [CrossRef]
- Pötter, M.; Dehne, H.; Reinke, H.; Dobbertin, J.; Schick, C. Glass-Forming Terephthalic Esters with Lateral Phenylthio Groups and Their Relaxation Behavior. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1998, 312, 55–68. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, P.; Ren, J.; Chen, Y.; Li, X.; Sha, J.; Jiang, J. Surfactant-Assisted Synthesis and Electrochemical Properties of an Unprecedented Polyoxometalate-Based Metal-Organic Nanocaged Framework. Chem. Commun. 2019, 55, 1201–1204. [Google Scholar] [CrossRef]
- Wu, Y.; Kobayashi, A.; Halder, G.J.; Peterson, V.K.; Chapman, K.W.; Lock, N.; Southon, P.D.; Kepert, C.J. Negative Thermal Expansion in the Metal-Organic Framework Material Cu 3(1,3,5-Benzenetricarboxylate)2. Angew. Chem. Int. Ed. 2008, 47, 8929–8932. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Yang, H.M.; Liu, X.; Song, X.L.; Yang, T.L.; Liang, Z.H.; Fan, C.M. In Situ Electrochemical Synthesis of MOF-5 and Its Application in Improving Photocatalytic Activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- Martinez Joaristi, A.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Zhu, Q.; Dong, R.; Yang, R.; Cheng, Y.; He, C. Microwave-Assisted Rapid Synthesis of Well-Shaped MOF-74 (Ni) for CO2 Efficient Capture. Inorg. Chem. 2019, 58, 2717–2728. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid Production of Metal-Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Zhang, W.X.; Yang, Y.Y.; Zai, S.B.; Seik, W.N.; Chen, X.M. Syntheses, Structures and Magnetic Properties of Dinuclear Copper(II)-Lanthanide(III) Complexes Bridged by 2-Hydroxymethyl-1- Methylimidazole. Eur. J. Inorg. Chem. 2008, 2, 679–685. [Google Scholar] [CrossRef]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochemistry. Angew. Chem. 2010, 122, 9834–9837. [Google Scholar] [CrossRef]
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-Free Synthesis of Metal Complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The Varied Applications of Mechanical Bond-Breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Mason, T.J. Ultrasound in Synthetic Organic Chemistry. Chem. Soc. Rev. 1997, 26, 443–451. [Google Scholar] [CrossRef]
- Yu, K.; Lee, Y.R.; Seo, J.Y.; Baek, K.Y.; Chung, Y.M.; Ahn, W.S. Sonochemical Synthesis of Zr-Based Porphyrinic MOF-525 and MOF-545: Enhancement in Catalytic and Adsorption Properties. Microporous Mesoporous Mater. 2021, 316, 110985. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarandi, M.B.; Nateghi, M.R. Highly Efficient Removal of Dye Pollutants by MIL-101(Fe) Metal-Organic Framework Loaded Magnetic Particles Mediated by Poly L-Dopa. J. Environ. Chem. Eng. 2019, 7, 102882. [Google Scholar] [CrossRef]
- Tian, M.; Pei, F.; Yao, M.; Fu, Z.; Lin, L.; Wu, G.; Xu, G.; Kitagawa, H.; Fang, X. Ultrathin MOF Nanosheet Assembled Highly Oriented Microporous Membrane as an Interlayer for Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 21, 14–21. [Google Scholar] [CrossRef]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Fraser Stoddart, J.; Gref, R.; et al. Composite CD-MOF Nanocrystals-Containing Microspheres for Sustained Drug Delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [Green Version]
- De Lima Neto, O.J.; Frós, A.C.d.O.; Barros, B.S.; De Farias Monteiro, A.F.; Kulesza, J. Rapid and Efficient Electrochemical Synthesis of a Zinc-Based Nano-MOF for Ibuprofen Adsorption. New J. Chem. 2019, 43, 5518–5524. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. Microwave Synthesis and Characterization of MOF-74 (M = Ni, Mg) for Gas Separation. Microporous Mesoporous Mater. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Lu, C.M.; Liu, J.; Xiao, K.; Harris, A.T. Microwave Enhanced Synthesis of MOF-5 and Its CO2 Capture Ability at Moderate Temperatures across Multiple Capture and Release Cycles. Chem. Eng. J. 2010, 156, 465–470. [Google Scholar] [CrossRef]
- Javanbakht, S.; Nezhad-Mokhtari, P.; Shaabani, A.; Arsalani, N.; Ghorbani, M. Incorporating Cu-Based Metal-Organic Framework/Drug Nanohybrids into Gelatin Microsphere for Ibuprofen Oral Delivery. Mater. Sci. Eng. C 2019, 96, 302–309. [Google Scholar] [CrossRef]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. Alginate-Coated ZIF-8 Metal-Organic Framework as a Green and Bioactive Platform for Controlled Drug Release. J. Drug Deliv. Sci. Technol. 2019, 49, 570–576. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a Nanostructured Pillar MOF with High Adsorption Capacity towards Antibiotics Pollutants from Aqueous Solution. J. Hazard Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.H. Metal Organic Frameworks for Sensing Applications. TrAC Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF Positioning Technology and Device Fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [Green Version]
- Yap, M.H.; Fow, K.L.; Chen, G.Z. Synthesis and Applications of MOF-Derived Porous Nanostructures. Green Energy Environ. 2017, 2, 218–245. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and More: Concepts, Properties and Applications for Porous Coordination Networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Usman, M.; Mendiratta, S.; Lu, K.L. Semiconductor Metal–Organic Frameworks: Future Low-Bandgap Materials. Adv. Mater. 2017, 29, 1–5. [Google Scholar] [CrossRef]
- Lv, K.; Fichter, S.; Gu, M.; März, J.; Schmidt, M. An Updated Status and Trends in Actinide Metal-Organic Frameworks (An-MOFs): From Synthesis to Application. Coord. Chem. Rev. 2021, 446, 214011. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, K.; Tian, J.; Liu, C.; Tian, J.; Jiang, F.; Yuan, D.; Zhang, J.; Chen, Q.; Hong, M. Induction of Chirality in a Metal–Organic Framework Built from Achiral Precursors. Angew. Chem. 2021, 133, 3124–3131. [Google Scholar] [CrossRef]
- Cheng, R.; Li, W.; Wei, W.; Huang, J.; Li, S. Molecular Insights into the Correlation between Microstructure and Thermal Conductivity of Zeolitic Imidazolate Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 14141–14149. [Google Scholar] [CrossRef]
- Kolobov, N.; Goesten, M.G.; Gascon, J. Metal–Organic Frameworks: Molecules or Semiconductors in Photocatalysis? Angew. Chem. Int. Ed. 2021, 60, 26038–26052. [Google Scholar] [CrossRef]
- Yu, F.; Bai, X.; Liang, M.; Ma, J. Recent Progress on Metal-Organic Framework-Derived Porous Carbon and Its Composite for Pollutant Adsorption from Liquid Phase. Chem. Eng. J. 2021, 405, 126960. [Google Scholar] [CrossRef]
- Younis, S.A.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.H.; Deep, A. Rare Earth Metal–Organic Frameworks (RE-MOFs): Synthesis, Properties, and Biomedical Applications. Coord. Chem. Rev. 2021, 429, 213620. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, M.; Liu, C.; Li, S.; Li, Z.; Jiang, F.; Chen, L.; Hong, M. Tunable Dual-Emission Luminescence from Cu(i)-Cluster-Based MOFs for Multi-Stimuli Responsive Materials. J. Mater. Chem. C 2021, 9, 2890–2897. [Google Scholar] [CrossRef]
- Ma, L.L.; Yang, G.P.; Li, G.P.; Zhang, P.F.; Jin, J.; Wang, Y.; Wang, J.M.; Wang, Y.Y. Luminescence Modulation, near White Light Emission, Selective Luminescence Sensing, and Anticounterfeiting: Via a Series of Ln-MOFs with a p-Conjugated and Uncoordinated Lewis Basic Triazolyl Ligand. Inorg. Chem. Front. 2021, 8, 329–338. [Google Scholar] [CrossRef]
- Pan, S.; Chen, X.; Li, X.; Jin, M. Nonderivatization Method for Determination of Glyphosate, Glufosinate, Bialaphos, and Their Main Metabolites in Environmental Waters Based on Magnetic Metal-Organic Framework Pretreatment. J. Sep. Sci. 2019, 42, 1045–1050. [Google Scholar] [CrossRef]
- Ghanem, A.; Bados, P.; Kerhoas, L.; Dubroca, J.; Einhorn, J. Glyphosate and AMPA Analysis in Sewage Sludge by LC-ESI-MS/MS after FMOC Derivatization on Strong Anion-Exchange Resin as Solid Support. Anal. Chem. 2007, 79, 3794–3801. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water Adsorption in MOFs: Fundamentals and Applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef] [Green Version]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of Heavy Metal Ions from Wastewater by Chemically Modified Plant Wastes as Adsorbents: A Review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive Removal of Hazardous Materials Using Metal-Organic Frameworks (MOFs): A Review. J. Hazard Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon Dioxide Removal through Physical Adsorption Using Carbonaceous and Non-Carbonaceous Adsorbents: A Review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Wang, H.; Lou, X.; Hu, Q.; Sun, T. Adsorption of Antibiotics from Water by Using Chinese Herbal Medicine Residues Derived Biochar: Preparation and Properties Studies. J. Mol. Liq. 2021, 325, 2–10. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme Encapsulation in Metal-Organic Frameworks for Applications in Catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous Removal of Tetracycline and Oxytetracycline Antibiotics from Wastewater Using a ZIF-8 Metal Organic-Framework. J. Hazard Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Jang, M.; Park, C.M.; Muñoz-Senmache, J.C.; Hernández-Maldonado, A.J.; Heyden, A.; Yu, M.; Yoon, Y. Removal of Contaminants of Emerging Concern by Metal-Organic Framework Nanoadsorbents: A Review. Chem. Eng. J. 2019, 369, 928–946. [Google Scholar] [CrossRef]
- Dhankhar, S.S.; Nagaraja, C.M. Construction of a 3D Porous Co(Ii) Metal-Organic Framework (MOF) with Lewis Acidic Metal Sites Exhibiting Selective CO2 Capture and Conversion under Mild Conditions. New J. Chem. 2019, 43, 2163–2170. [Google Scholar] [CrossRef]
- Huelsenbeck, L.; Westendorff, K.S.; Gu, Y.; Marino, S.; Jung, S.; Epling, W.S.; Giri, G. Modulating and Orienting an Anisotropic Zn-Based Metal Organic Framework for Selective CH4/CO2 Gas Separation. Crystals 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Ikreedeegh, R.R.; Tahir, M. A Critical Review in Recent Developments of Metal-Organic-Frameworks (MOFs) with Band Engineering Alteration for Photocatalytic CO2 reduction to Solar Fuels. J. CO2 Util. 2021, 43, 101381. [Google Scholar] [CrossRef]
- Wang, J.; Cherevan, A.S.; Hannecart, C.; Naghdi, S.; Nandan, S.P.; Gupta, T.; Eder, D. Ti-Based MOFs: New Insights on the Impact of Ligand Composition and Hole Scavengers on Stability, Charge Separation and Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2021, 283, 119626. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Ricoux, R.; Kariyawasam, K.; Stenger, I.; Fan, B.; Ayoub, N.; Salas, S.; Patriarche, G.; Serre, C.; Mahy, J.P.; et al. Encapsulation of Microperoxidase-8 in MIL-101(Cr)-X Nanoparticles: Influence of Metal-Organic Framework Functionalization on Enzymatic Immobilization and Catalytic Activity. ACS Appl. Nano Mater. 2020, 3, 3233–3243. [Google Scholar] [CrossRef]
- Phipps, J.; Chen, H.; Donovan, C.; Dominguez, D.; Morgan, S.; Weidman, B.; Fan, C.; Fan, C.; Beyzavi, M.H.; Beyzavi, M.H. Catalytic Activity, Stability, and Loading Trends of Alcohol Dehydrogenase Enzyme Encapsulated in a Metal-Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 26084–26094. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of Biomolecules in Metal-Organic Frameworks for Advanced Applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Li, B.; Suo, T.; Xie, S.; Xia, A.; Ma, Y.J.; Huang, H.; Zhang, X.; Hu, Q. Rational Design, Synthesis, and Applications of Carbon Dots@metal–Organic Frameworks (CD@MOF) Based Sensors. TrAC Trends Anal. Chem. 2021, 135, 116163. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, J.; Pang, H. Metal-Organic Framework-Based Materials as an Emerging Platform for Advanced Electrochemical Sensing. Coord. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Huang, L.; Zhang, Z.; Dong, S. GOx@ZIF-8(NiPd) Nanoflower: An Artificial Enzyme System for Tandem Catalysis. Angew. Chem. 2017, 129, 16298–16301. [Google Scholar] [CrossRef]
- Kornienko, N. Operando Spectroscopy of Nanoscopic Metal/Covalent Organic Framework Electrocatalysts. Nanoscale 2021, 13, 1507–1514. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, S.; Qian, G. Metal-Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef]
- Wang, Z.; Gui, M.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A Facile Modular Approach to the 2D Oriented Assembly MOF Electrode for Non-Enzymatic Sweat Biosensors. Nanoscale 2018, 10, 6629–6638. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Wang, Y.; Ying, Y. Recent Advances in the Rational Synthesis and Sensing Applications of Metal-Organic Framework Biocomposites. Coord. Chem. Rev. 2019, 387, 60–78. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Zhu, P.; Tian, Y.; Chen, Y.; Wu, C. Applications of Functional Metal-Organic Frameworks in Biosensors. Biotechnol. J. 2021, 16, 1–12. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Z.; Jin, H.; Gui, R. Urate Oxidase-Loaded MOF Electrodeposited on Boron Nanosheet-Doxorubicin Complex as Multifunctional Nano-Enzyme Platform for Enzymatic and Ratiometric Electrochemical Biosensing. Talanta 2022, 243, 123359. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, F.; Sun, Y.; Yu, K.; Guo, W.; Qu, F. A 2D/2D NiCo-MOF/Ti3C2 heterostructure for the Simultaneous Detection of Acetaminophen, Dopamine and Uric Acid by Differential Pulse Voltammetry. Dalt. Trans. 2021, 50, 16593–16600. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Ruthenium-Based Conjugated Polymer and Metal-Organic Framework Nanocomposites for Glucose Sensing. Electroanalysis 2021, 33, 1902–1910. [Google Scholar] [CrossRef]
- Bao, T.; Fu, R.; Jiang, Y.; Wen, W.; Zhang, X.; Wang, S. Metal-Mediated Polydopamine Nanoparticles-DNA Nanomachine Coupling Electrochemical Conversion of Metal-Organic Frameworks for Ultrasensitive MicroRNA Sensing. Anal. Chem. 2021, 93, 13475–13484. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Zhang, S.; Tian, Q.; Liu, Y.; Zhang, P.; Wang, M.; Zhang, J.; Li, X. Cu-MOF/Hemin: A Bionic Enzyme with Excellent Dispersity for the Determination of Hydrogen Peroxide Released from Living Cells. Analyst 2021, 146, 5951–5961. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework for Ultrasensitive Immobilization-Free Photoelectrochemical Biosensing. Biosens. Bioelectron. 2021, 173, 112832. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1–24. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Ji, X.; Mo, Y.; Li, H.; Zhao, W.; Zhong, A.; Li, S.; Wang, Q.; Duan, X.; Xiao, J. Gender-Dependent Reproductive Toxicity of Copper Metal-Organic Frameworks and Attenuation by Surface Modification. Nanoscale 2021, 13, 7389–7402. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, R.; Hou, C.; Wang, Z. Zn–Porphyrin Metal–Organic Framework–Based Photoelectrochemical Enzymatic Biosensor for Hypoxanthine. J. Solid State Electrochem. 2022, 26, 565–572. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Xiong, C.; Zhu, X.; Chen, C.; Zhou, F.; Dong, Y.; Wang, Y.; Xu, J.; Li, Y.; et al. Dual Confinement of High–Loading Enzymes within Metal–Organic Frameworks for Glucose Sensor with Enhanced Cascade Biocatalysis. Biosens. Bioelectron. 2022, 196, 113695. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Gao, Z.; Zhao, C.; Mei, Y.; Song, Y.Y. Insight of MOF Environment-Dependent Enzyme Activity via MOFs-in-Nanochannels Configuration. ACS Catal. 2020, 10, 5949–5958. [Google Scholar] [CrossRef]
- Kustov, L.M.; Isaeva, V.I.; Přech, J.; Bisht, K.K. Metal-Organic Frameworks as Materials for Applications in Sensors. Mendeleev Commun. 2019, 29, 361–368. [Google Scholar] [CrossRef]
- Zhou, H.C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanpour, M.; Bhargava, P.; Varma, A.; Choudhary, D.K. Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer: Singapore, 2020; ISBN 9789811529856. [Google Scholar]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A Review on Current Trends in Potential Use of Metal-Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen Storage in Metal-Organic Frameworks: A Review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef]
- Ma, L.; He, Y.; Wang, Y.; Wang, Y.; Li, R.; Huang, Z.; Jiang, Y.; Gao, J. Nanocomposites of Pt Nanoparticles Anchored on UiO66-NH2 as Carriers to Construct Acetylcholinesterase Biosensors for Organophosphorus Pesticide Detection. Electrochim. Acta 2019, 318, 525–533. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic Gas Removal-Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, G.; Peh, S.B.; Zhao, D.; Ho, G.W. Atomic- and Molecular-Level Design of Functional Metal–Organic Frameworks (MOFs) and Derivatives for Energy and Environmental Applications. Adv. Sci. 2019, 6, 1901129. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and Characterization of an SWCNT@HKUST-1 Composite: Enhancing the CO2 Adsorption Properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.; Dhaka, S.; Paul, A.K.; Dayananda, S.; Deep, A. Organophosphate Hydrolase Conjugated UiO-66-NH2 MOF Based Highly Sensitive Optical Detection of Methyl Parathion. Environ. Res. 2019, 174, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal-Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Ying, R.J.; Han, C.X.; Hu, Q.T.; Xu, H.M.; Li, J.H.; Wang, Q.; Zhang, W. Adsorptive Removal of Organic Dyes from Aqueous Solution by a Zr-Based Metal-Organic Framework: Effects of Ce(III) Doping. Dalt. Trans. 2018, 47, 3913–3920. [Google Scholar] [CrossRef]
- Yu, L.L.; Luo, Z.F.; Zhang, Y.Y.; Wu, S.C.; Yang, C.; Cheng, J. hua Contrastive Removal of Oxytetracycline and Chlortetracycline from Aqueous Solution on Al-MOF/GO Granules. Environ. Sci. Pollut. Res. 2019, 26, 3685–3696. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, G.; Song, Z.; Zhou, Y.P.; Chao, H.Y.; Yuan, D.; Tan, T.T.Y.; Guo, Z.; Hu, Z.; Tang, B.Z.; et al. Two-Dimensional Metal-Organic Framework with Wide Channels and Responsive Turn-on Fluorescence for the Chemical Sensing of Volatile Organic Compounds. J. Am. Chem. Soc. 2014, 136, 7241–7244. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Qi, S.; Chen, Y.; Yin, M.; Zhang, L.; Ge, K.; Wei, X.; Tian, X.; Wang, P.; Li, M.; et al. Hierarchical Mesoporous Hollow Ce-MOF Nanosphere as Oxidase Mimic for Highly Sensitive Colorimetric Detection of Ascorbic Acid. Chem. Phys. Lett. 2021, 777, 138749. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Sharmoukh, W. Intrinsic Catalase-Mimicking MOFzyme for Sensitive Detection of Hydrogen Peroxide and Ferric Ions. Microchem. J. 2021, 163, 105873. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, S.; Liu, M.D.; Yu, W.Y.; Zhang, M.K.; Zhang, L.; Zeng, X.; Zhang, X.Z. PH-Sensitive MOF Integrated with Glucose Oxidase for Glucose-Responsive Insulin Delivery. J. Control. Release 2020, 320, 159–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, H.C. Direct Synthesis of Functionalized PCN-333 via Linker Design for Fe3+ Detection in Aqueous Media. Dalt. Trans. 2018, 47, 11806–11811. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zha, Q.; Zhai, C.; Li, W.; Zeng, L.; Zhu, M. Photo-Electrochemical Detection of Dopamine in Human Urine and Calf Serum Based on MIL-101 (Cr)/Carbon Black. Microchim. Acta 2020, 187, 526. [Google Scholar] [CrossRef]
- Haghighi, E.; Zeinali, S. Nanoporous MIL-101(Cr) as a Sensing Layer Coated on a Quartz Crystal Microbalance (QCM) Nanosensor to Detect Volatile Organic Compounds (VOCs). RSC Adv. 2019, 9, 24460–24470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, B.; Guo, P.; Yang, M.; Ma, Y.; Yan, X.; Jia, Z.; Gao, W.; Ahmad, S.; Xu, C.; Liu, C.; et al. A Novel Fluorescent Enhancing Platform Based on DNA-Scaffolded Silver Nanoclusters for Potential Inflammatory Bowel Disease-Associated MicroRNA Detection. Talanta 2020, 218, 121122. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, J.; Khataee, A.; Eskandari, H. Encapsulated Cholesterol Oxidase in Metal-Organic Framework and Biomimetic Ag Nanocluster Decorated MoS2 Nanosheets for Sensitive Detection of Cholesterol. Sens. Actuators B Chem. 2018, 259, 402–410. [Google Scholar] [CrossRef]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/Carbon Nanohorn/β-Cyclodextrin-Metal-Organic Frameworks as High-Performance Electrochemical Sensing Platform for Sensitive Detection of Carbendazim Pesticide. J. Hazard Mater. 2020, 396, 122776. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, S.; Wang, M.; Xie, X.; Su, X. Self-Assembled Dual-Emissive Nanoprobe with Metal−organic Frameworks as Scaff; Olds for Enhanced Ascorbic Acid and Ascorbate Oxidase Sensing. Sens. Actuators B Chem. 2021, 339, 129910. [Google Scholar] [CrossRef]
- Yu, J.; Wei, Z.; Li, Q.; Wan, F.; Chao, Z.; Zhang, X.; Lin, L.; Meng, H.; Tian, L. Advanced Cancer Starvation Therapy by Simultaneous Deprivation of Lactate and Glucose Using a MOF Nanoplatform. Adv. Sci. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Moghaddam, Z.S.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Synthesis of UiO-66-OH Zirconium Metal-Organic Framework and Its Application for Selective Extraction and Trace Determination of Thorium in Water Samples by Spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 76–82. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Encapsulation of Lipase within Metal-Organic Framework (MOF) with Enhanced Activity Intensified under Ultrasound. Enzym. Microb. Technol. 2018, 108, 11–20. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; Li, C.; Gu, J. In Situ Coupling of Catalytic Centers into Artificial Substrate Mesochannels as Super-Active Metalloenzyme Mimics. Small 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Zhou, J.; Long, Z.; Tian, Y.; Ding, X.; Wu, L.; Hou, X. A Chemiluminescence Metalloimmunoassay for Sensitive Detection of Alpha-Fetoprotein in Human Serum Using Fe-MIL-88B-NH2 as a Label. Appl. Spectrosc. Rev. 2016, 51, 517–526. [Google Scholar] [CrossRef]
- Lykourinou, V.; Chen, Y.; Wang, X.; Meng, L.; Hoang, T.; Ming, L.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a Mesoporous Metal?Organic Framework, MP-11@mesoMOF: A New Platform for Enzymatic Catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Cheng, L.; Qi, R.; Zhang, M.; Zhao, J.; Zhu, L.; Dong, M. A Metal-Organic Zeolitic Framework with Immobilized Urease for Use in a Tapered Optical Fiber Urea Biosensor. Microchim. Acta 2020, 187, 72. [Google Scholar] [CrossRef]
- Liu, W.L.; Lo, S.H.; Singco, B.; Yang, C.C.; Huang, H.Y.; Lin, C.H. Novel Trypsin-FITC@MOF Bioreactor Efficiently Catalyzes Protein Digestion. J. Mater. Chem. B 2013, 1, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, T.; Du, Y.; Ding, W.; Chen, C.; Ma, X. Correction to: Metal Organic Framework HKUST-1 Modified with Carboxymethyl-β-Cyclodextrin for Use in Improved Open Tubular Capillary Electrochromatographic Enantioseparation of Five Basic Drugs (Microchimica Acta, (2019), 186, 7, (462), 10.1007/S00604-019. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, F.; Zhang, S.; Cai, Y.; Cao, S.; Li, S.; Zhao, W.; Yuan, S.; Feng, X.; Cao, A.; et al. Facile Fabrication of Multifunctional Metal-Organic Framework Hollow Tubes to Trap Pollutants. J. Am. Chem. Soc. 2017, 139, 16482–16485. [Google Scholar] [CrossRef]
- Chen, S.; Wen, L.; Svec, F.; Tan, T.; Lv, Y. Magnetic Metal-Organic Frameworks as Scaffolds for Spatial Co-Location and Positional Assembly of Multi-Enzyme Systems Enabling Enhanced Cascade Biocatalysis. RSC Adv. 2017, 7, 21205–21213. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and Applications of Metal-Organic Framework-Quantum Dot (QD at MOF) Composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, N.; Zhang, J.; Zhang, Z.; Dang, F. Size-Selective QD@MOF Core-Shell Nanocomposites for the Highly Sensitive Monitoring of Oxidase Activities. Biosens. Bioelectron. 2017, 87, 339–344. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q. Application of QD-MOF Composites for Photocatalysis: Energy Production and Environmental Remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Yang, G.L.; Jiang, X.L.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 1–19. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A Novel Electrochemical Sensor for Glucose Detection Based on Ag@ZIF-67 Nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Aguado, S.; Quirós, J.; Canivet, J.; Farrusseng, D.; Boltes, K.; Rosal, R. Antimicrobial Activity of Cobalt Imidazolate Metal-Organic Frameworks. Chemosphere 2014, 113, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Sensitive Biosensing of Organophosphate Pesticides Using Enzyme Mimics of Magnetic ZIF-8. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Gascón, V.; Jiménez, M.B.; Blanco, R.M.; Sanchez-Sanchez, M. Semi-Crystalline Fe-BTC MOF Material as an Efficient Support for Enzyme Immobilization. Catal. Today 2018, 304, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Girão Neto, C.A.C.; Silva, N.C.G.E.; de Oliveira Costa, T.; de Albuquerque, T.L.; Gonçalves, L.R.B.; Fernandez-Lafuente, R.; Rocha, M.V.P. The β-Galactosidase Immobilization Protocol Determines Its Performance as Catalysts in the Kinetically Controlled Synthesis of Lactulose. Int. J. Biol. Macromol. 2021, 176, 468–478. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-Metal Organic Framework (Magnetic-MOF): A Novel Platform for Enzyme Immobilization and Nanozyme Applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Rathod, V.K. Enzyme Embedded Metal Organic Framework (Enzyme–MOF): De Novo Approaches for Immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Yu, F. Facile Preparation of Fe3O4@MOF Core-Shell Microspheres for Lipase Immobilization. J. Taiwan Inst. Chem. Eng. 2016, 69, 139–145. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green Synthesis of Enzyme/Metal-Organic Framework Composites with High Stability in Protein Denaturing Solvents. Bioresour. Bioprocess 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Gascón, V.; Castro-Miguel, E.; Díaz-García, M.; Blanco, R.M.; Sanchez-Sanchez, M. In Situ and Post-Synthesis Immobilization of Enzymes on Nanocrystalline MOF Platforms to Yield Active Biocatalysts. J. Chem. Technol. Biotechnol. 2017, 92, 2583–2593. [Google Scholar] [CrossRef]
- Cui, J.; Ren, S.; Sun, B.; Jia, S. Optimization Protocols and Improved Strategies for Metal-Organic Frameworks for Immobilizing Enzymes: Current Development and Future Challenges. Coord. Chem. Rev. 2018, 370, 22–41. [Google Scholar] [CrossRef]
- Cao, S.L.; Yue, D.M.; Li, X.H.; Smith, T.J.; Li, N.; Zong, M.H.; Wu, H.; Ma, Y.Z.; Lou, W.Y. Novel Nano-/Micro-Biocatalyst: Soybean Epoxide Hydrolase Immobilized on UiO-66-NH2 MOF for Efficient Biosynthesis of Enantiopure (R)-1, 2-Octanediol in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2016, 4, 3586–3595. [Google Scholar] [CrossRef]
- Pang, S.; Wu, Y.; Zhang, X.; Li, B.; Ouyang, J.; Ding, M. Immobilization of Laccase via Adsorption onto Bimodal Mesoporous Zr-MOF. Process Biochem. 2016, 51, 229–239. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, Z.; Wang, T.; Xiao, Y.; Huo, Q.; Liu, Y. Immobilization of: Bacillus Subtilis Lipase on a Cu-BTC Based Hierarchically Porous Metal-Organic Framework Material: A Biocatalyst for Esterification. Dalt. Trans. 2016, 45, 6998–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Moon, S.Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a Nerve Agent Detoxifying Enzyme by a Mesoporous Zirconium Metal-Organic Framework Engenders Thermal and Long-Term Stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef]
- Lian, X.; Chen, Y.P.; Liu, T.F.; Zhou, H.C. Coupling Two Enzymes into a Tandem Nanoreactor Utilizing a Hierarchically Structured MOF. Chem. Sci. 2016, 7, 6969–6973. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tu, R.; Lu, Z.; Peng, J.; Hou, C.; Wang, Z. Hierarchical Mesoporous Metal–Organic Frameworks Encapsulated Enzymes: Progress and Perspective. Coord. Chem. Rev. 2021, 443, 214032. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Limareva, L.V.; Iliasov, P.V.; Gribkova, O.V.; Sustretov, A.S. Methods of Encapsulation of Biomacromolecules and Living Cells. Prospects of Using Metal–Organic Frameworks. Russ. J. Org. Chem. 2021, 57, 491–505. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, L.; Wu, Y.; Hu, L.; Gu, W.; Zhu, C. Metal–Organic Frameworks Enhance Biomimetic Cascade Catalysis for Biosensing. Adv. Mater. 2021, 33, 1–18. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Simpson, B. Food Enzymes Immobilization: Novel Carriers, Techniques and Applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]










| Synthesis Strategy | Main Features | Applications | Material | Ref. |
|---|---|---|---|---|
| Hydrothermal (Solvothermal) | Generally, processes that use water as a solvent are termed hydrothermal processes, while those that use other solvents are classified as solvothermal processes [124,126] | Dye removal | MIL-101(Fe)@PDopa@Fe3O4 | [144] |
| Lithium–sulfur battery | Cu2(CuTCPP) | [145] | ||
| Diffusion | Diffusion MOF synthesis methods can be subdivided into two strategies: diffusion between two liquids with different densities (no physical barrier) and gradual diffusion that occurs through physical barriers [111] | Drug delivery | CD-MOF | [146] |
| Adsorption of copper ions | MOF-5 | [112] | ||
| Electrochemical | Electrochemical synthesis is widely used at an industrial scale to produce MOFs [129] | Lithium-ion batteries | Zn-POMCF | [129] |
| Ibuprofen adsorption | [Zn(1,3-bdc)0.5(bzim)] | [147] | ||
| Microwave-assisted | The microwave technique is widely used in synthesizing small particles of oxides and metals [137] | Gas separation | MOF-74 | [148] |
| CO2 capture | MOF-5 | [149] | ||
| Mechanochemical | In this type of synthesis, chemical transformation is preceded by the mechanical rupture of intermolecular bonds [139,140] | Drug delivery | Cu-MOF/IBU@GM | [150] |
| Drug delivery | ZIF-8@alginate NPs | [151] | ||
| Sonochemistry | This methodology uses frequencies between 10 MHz and 20 KHz, which are higher than those detectable by the human ear, for dispersion and agglomeration purposes [102] | Adsorption of antibiotics | [Zn6(IDC)4(OH)2(Hprz)2]n | [152] |
| DMNP hydrolysis and BPA adsorption | MOF-525 and MOF-545 | [143] |
| N0 | MOFs | Enzyme | Applications | Ref. |
|---|---|---|---|---|
| 1 | ZIF-90/Ce-MOF | Catalase | Sensitive detection and degradation of hydrogen peroxide | [219,220] |
| 2 | L-MOFs | Glucose oxidase | Insulin delivery | [85,221] |
| 3 | PCN-333(Fe) | Alcohol Dehydrogenase | Catalysis of the conversion of toxic levels of alcohols to aldehydes in cells | [181,222] |
| 4 | MIL-101(Cr) | Microperoxidase 8 | Dual catalytic activity in the selective oxidation of organic molecules | [180,223,224] |
| 5 | AgNC/Mo(II)-NS | Cholesterol oxidase | Detection and concentration in blood vessels or other body tissues | [225,226] |
| 6 | QDs/CDs@MOFs | Ascorbate oxidase | Improved ascorbic acid detection | [227,228] |
| 7 | ZIF-8 | Lactate/glucose oxidase | Tumor cell mapping and energy reduction for tumor cycles | [229] |
| 8 | UiO-66 | Lipase | Drug synthesis against venous thromboembolism | [230,231] |
| 9 | OMUiO-66 (Ce) | Glutamate oxidase | Screening of specific chiral amino acids in complex biological samples | [198,232] |
| 10 | ZIF-8 | Glucose oxidase | Electrochemical glucose detection | [186] |
| 11 | MIL-88B-NH2(Cr) | Trypsin | Protein degradation by enzymatic hydrolysis | [99,233] |
| 12 | ZIF-8 | Glucose oxidase | Electrochemical glucose detection | [99] |
| 13 | Tb-mesoMOF | Mb | Oxidation of ABTS and THB | [99,234] |
| 14 | ZIF-8 | Urease | Sensitive biosensor for urea detection | [235] |
| 15 | CYCU-4 | Trypsin | Protein digestion | [99,236] |
| 16 | HKUST-1 | Peroxidase | CO2 adsorption | [99,213,237] |
| 17 | UIO66-NH2 | Acetylcholinesterase | Biosensors for organophosphorus pesticide detection | [166,210] |
| 18 | MOF-199 | Laccase | Removal of heavy metals from fluids and aquatic environments | [238,239] |
| 19 | QD-MOF | Oxidase | Degradation of organic dyes in industrial wastewaters | [240,241,242] |
| 20 | L-MOFs | Lipase | Luminescent sensors for environmental pollutants | [125,243] |
| 21 | ZIF-90 | Catalase | Effluent treatment in wastewater | [214,216,244] |
| 22 | ZIF-67 | Glucose oxidase | Antimicrobial agent | [244,245] |
| 23 | Ce (III)/UiO-66 | Hydrolases | Adsorptive removal of organic dyes from aqueous solutions | [214,216] |
| 24 | ZIF-8 | Choline oxidase | Detection and removal of water pollutants | [215,246] |
| Strategy | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| In situ synthesis | Easily conducted; requires only mild reaction conditions | Not all MOFs are ideal candidates to the process | [252] |
| Covalent bonding | The enzyme is strongly attached to the surface of the support; several MOFs can be used | It can change the morphology of the enzyme, altering its activity or even inactivating it | [93,251] |
| Surface immobilization (adsorption) | Relative low cost and simple methodology | Enzymes can be easily leached from supports due to variations in pH and temperature | [93,254] |
| Entrapment | Gives proteins greater stability against denaturation caused by organic solvents, high temperatures, or sudden changes in pH | Mass transfer limitations may occur; difficult for substrates to reach the active site of enzymes | [93,173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Neto, J.G.L.; de Sousa Júnior, P.G.; Rocha, M.V.P.; dos Santos, J.C.S. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. https://doi.org/10.3390/molecules27144529
Silva ARM, Alexandre JYNH, Souza JES, Neto JGL, de Sousa Júnior PG, Rocha MVP, dos Santos JCS. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules. 2022; 27(14):4529. https://doi.org/10.3390/molecules27144529
Chicago/Turabian StyleSilva, Allison R. M., Jeferson Y. N. H. Alexandre, José E. S. Souza, José G. Lima Neto, Paulo G. de Sousa Júnior, Maria V. P. Rocha, and José C. S. dos Santos. 2022. "The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems" Molecules 27, no. 14: 4529. https://doi.org/10.3390/molecules27144529