Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Rooibos and Reagents
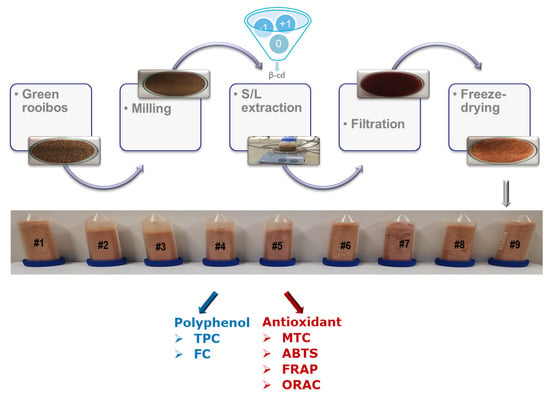
2.2. Solid-Liquid Extraction of Green Rooibos
2.3. Total Polyphenolic Content (TPC) and Quantification of Selected Flavonoids
2.4. Metal Chelation
2.5. Ferric Reducing Antioxidant Power (FRAP)
2.6. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Scavenging
2.7. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.8. Experimental Design and Statistical Analysis
3. Results and Discussions
3.1. Fitting the Model
| Total Polyphenolic Content R2 = 0.8163 | Adjusted R2adj = 0.7924 | ||||
| Source | SS | DF | MS | f-Value | p-Value |
| Model | 38,588.59 | 3 | 12,862.86 | 34.07 | <0.0001 |
| β-CD | 37,567.44 | 1 | 37,567.44 | 99.51 | <0.0001 |
| Temperature | 434.58 | 1 | 434.58 | 1.15 | 0.2944 |
| Time | 586.57 | 1 | 586.57 | 1.55 | 0.2251 |
| Lack of fit | 1970.62 | 5 | 394.12 | 1.06 | 0.4159 |
| Pure error | 6712.69 | 18 | 372.93 | ||
| Metal Chelation R2 = 0.8826 | R2adj = 0.8658 | ||||
| Source | SS | DF | MS | f-Value | p-Value |
| Model | 5155.77 | 3 | 1718.59 | 52.62 | <0.0001 |
| β-CD | 5046.53 | 1 | 5046.53 | 154.51 | <0.0001 |
| Temperature | 237.19 | 1 | 237.19 | 7.26 | 0.0136 |
| Time | 27.06 | 1 | 27.06 | 0.8286 | 0.3730 |
| Lack of fit | 86.70 | 5 | 17.34 | 0.46 | 0.7980 |
| Pure error | 599.19 | 16 | 37.45 | ||
| 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) R2 = 0.9106 | R2adj = 0.8989 | ||||
| Source | SS | DF | MS | f-Value | p-Value |
| Model | 1.05 × 106 | 3 | 3.51 × 105 | 78.09 | <0.0001 |
| β-CD | 9.69 × 105 | 1 | 9.9 × 105 | 215.14 | <0.0001 |
| Temperature | 33,354.30 | 1 | 33,354.30 | 7.40 | 0.0122 |
| Time | 52,806.68 | 1 | 52,806.68 | 11.72 | 0.0023 |
| Lack of fit | 50,643.50 | 5 | 10,128.70 | 3.44 | 0.0234 |
| Pure error | 52,975.45 | 18 | 2943.08 | ||
| Ferric Reducing Power R2 = 0.8757 | R2adj = 0.8263 | ||||
| Source | SS | DF | MS | f-Value | p-Value |
| Model | 9.01 × 105 | 3 | 3.0 × 105 | 67.62 | <0.0001 |
| β-CD | 8.29 × 105 | 1 | 8.27 × 105 | 259.96 | <0.0001 |
| Temperature | 23,025.92 | 1 | 23,025.92 | 9.08 | 0.0592 |
| Time | 49,683.13 | 1 | 49,683.13 | 2.90 | 0.0079 |
| Lack of fit | 42,846.9 | 5 | 8569.34 | 2.59 | 0.1859 |
| Pure error | 85,123.63 | 17 | 5007.27 | ||
| Oxygen Radical Scavenging Activity R2 = 0.9537 | R2adj = 0.9427 | ||||
| Source | SS | DF | MS | f-Value | p-Value |
| Model | 8.54 × 107 | 5 | 1.71 × 107 | 86.61 | <0.0001 |
| β-CD | 6.06 × 107 | 1 | 6.05 × 107 | 307.02 | <0.0001 |
| Temperature | 3.02 × 106 | 1 | 3.02 × 106 | 15.32 | 0.0008 |
| Time | 1.82 × 106 | 1 | 1.82 × 106 | 9.23 | 0.0062 |
| β-CD vs. temperature | 8.90 × 105 | 1 | 8.90 × 105 | 4.51 | 0.0457 |
| Temperature vs. time | 9.37 × 105 | 1 | 9.37 × 105 | 4.75 | 0.0408 |
| Lack of fit | 1.357 × 105 | 3 | 3.44 × 105 | 1.99 | 0.1513 |
| Pure error | 1.302 × 106 | 18 | 1.73 × 105 | ||
3.2. Total Polyphenolic Content and Quantification of Selected Flavonoids
3.3. Metal Chelation
3.4. 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Radical Scavenging
3.5. Ferric Reducing Antioxidant Power
3.6. Oxygen Radical Absorbance Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joubert, E.; De Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. South Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Samahlubi, S. Traditional Knowledge Associated with Rooibos and Honeybush Species in South Africa. 2014. Available online: https://www.dffe.gov.za/sites/default/files/reports/traditionalknowledge_rooibosandhoneybushspecies_report.pdf (accessed on 1 May 2022).
- Lawal, A.O.; Davids, L.M.; JMarnewick, L. Phytomedicine oxidative stress associated injury of diesel exhaust particles in human umbilical vein endothelial cells. Phytomedicine 2019, 59, 15298. [Google Scholar] [CrossRef]
- Monsees, T.; Opuwari, C. Effect of rooibos (Aspalathus linearis) on the female rat reproductive tract and liver and kidney functions in vivo. South Afr. J. Bot. 2016, 110, 208–215. [Google Scholar] [CrossRef]
- Damiani, E.; Carloni, P.; Rocchetti, G.; Senizza, B.; Tiano, L.; Joubert, E.; de Beer, D.; Lucini, L. Impact of Cold versus Hot Brewing on the Phenolic Profile and Antioxidant Capacity of Rooibos (Aspalathus linearis) Herbal Tea. Antioxidants 2019, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Ku, S.-K.; Kwak, S.; Kim, Y.; Bae, J.-S. Aspalathin and Nothofagin from Rooibos (Aspalathus linearis) Inhibits High Glucose-Induced Inflammation In Vitro and In Vivo. Inflammation 2014, 38, 445–455. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.-J.; Han, J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Patel, O.; Muller, C.; Joubert, E.; Louw, J.; Rosenkranz, B.; Awortwe, C. Inhibitory interactions of Aspalathus linearis (rooibos) extracts and compounds, aspalathin and Z-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid, on cytochromes metabolizing hypoglycemic and hypolipidemic drugs. Molecules 2016, 21, 1515. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, F.M. Traditional Medicine in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid.-Based Complementary Altern. Med. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- De Beer, D.; Tobin, J.; Walczak, B.; Van Der Rijst, M.; Joubert, E. Phenolic composition of rooibos changes during simulated fermentation: Effect of endogenous enzymes and fermentation temperature on reaction kinetics. Food Res. Int. 2019, 121, 185–196. [Google Scholar] [CrossRef]
- Miller, N. Green Rooibos Nutraceutical: Optimisation of Hot Water Extraction and Spray-Drying by Quality-by-Design Methodology; University of Stellenbosch: Stellenbosch, South Africa, 2016; Available online: http://hdl.handle.net/10019.1/100264 (accessed on 16 July 2021).
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Wang, Y.; Qin, Y.; Liu, B.; Bai, M. Two green approaches for extraction of dihydromyricetin from Chinese vine tea using β-Cyclodextrin-based and ionic liquid-based ultrasonic-assisted extraction methods. Food Bioprod. Process. 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Lloyd, P.J.; van Wyk, J. Introduction to Extraction in Food Processing. In Enhancing Extraction Processes in the Food Industry; Lebovka, N., Vorobiev, E., Chemat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–24. [Google Scholar]
- Hai, L.; Fang, Y.; Zhi, T.; Chang, R. Study on preparation of β-cyclodextrin encapsulation tea extract. Int. J. Biol. Macromol. 2011, 49, 561–566. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Crystallographic evidence for β-cyclodextrin inclusion complexation facilitating the improvement of antioxidant activity of tea (+)-catechin and (−)-epicatechin. Carbohydr. Polym. 2016, 140, 362–373. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Menexis, N.; Iakovidis, D.; Makris, D.P.; Goula, A. A Green Extraction Process to Recover Polyphenols from Byproducts of Hemp Oil Processing. Recycling 2018, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Ratnasooriya, C.C.; Rupasinghe, H.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Parmar, I.; Sharma, S.; Rupasinghe, H.P.V. Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. J. Food Sci. Technol. 2014, 52, 2202–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favre, L.C.; Rolandelli, G.; Mshicileli, N.; Vhangani, L.N.; Ferreira, C.I.d.S.; van Wyk, J.; Buera, M.D.P. Antioxidant and anti-glycation potential of green pepper (Piper nigrum): Optimization of β-cyclodextrin-based extraction by response surface methodology. Food Chem. 2020, 316, 126280. [Google Scholar] [CrossRef]
- García-Padial, M.; Martínez-Ohárriz, M.C.; Navarro-Blasco, I.; Zornoza, A. The role of cyclodextrins in ORAC-fluorescence assays. Antioxidant capacity of tyrosol and caffeic acid with hydroxypropyl-β-cyclodextrin. J. Agric. Food Chem. 2013, 61, 12260–12264. [Google Scholar] [CrossRef]
- Santos, J.S.; Deolindo, C.T.P.; Esmerino, L.A.; Genovese, M.I.; Fujita, A.; Marques, M.B.; Rosso, N.D.; Daguer, H.; Valese, A.C.; Granato, D. Effects of time and extraction temperature on phenolic composition and functional properties of red rooibos (Aspalathus linearis). Food Res. Int. 2016, 89, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Favre, L.C.; Santos, C.d.; López-Fernández, M.P.; Mazzobre, M.F.; Buera, M. Optimization of β-cyclodextrin-based extraction of antioxidant and anti-browning activities from thyme leaves by response surface methodology. Food Chem. 2018, 265, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, G.; Joubert, E.; VanZyl, W.H.; Viljoen-bloom, M. Food and Bioproducts Processing Improved extraction of phytochemicals from rooibos with enzyme treatment. Food Bioprod. Process. 2013, 92, 393–401. [Google Scholar] [CrossRef]
- Diamanti, A.C.; Igoumenidis, P.E.; Mourtzinos, I.; Yannakopoulou, K.; Karathanos, V.T. Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem. 2017, 214, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, S.; Jin, H.; Jiang, Y.; Xia, T. Effects of Additive β-Cyclodextrin on the Performances of Green Tea Infusion. J. Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pantsi, W.; Marnewick, J.L.; Esterhuyse, A.; Rautenbach, F.; van Rooyen, J. Rooibos (Aspalathus linearis) offers cardiac protection against ischaemia/reperfusion in the isolated perfused rat heart. Phytomedicine 2011, 18, 1220–1228. [Google Scholar] [CrossRef]
- Simpson, M.J.; Hjelmqvist, D.; López-Alarcón, C.; Karamehmedovic, N.; Minehan, T.G.; Yepremyan, A.; Salehani, B.; Lissi, E.; Joubert, E.; Udekwu, K.I.; et al. Anti-Peroxyl Radical Quality and Antibacterial Properties of Rooibos Infusions and Their Pure Glycosylated Polyphenolic Constituents. Molecules 2013, 18, 11264–11280. [Google Scholar] [CrossRef]
- Sinjman, P.W. Antioxidant activity of the dihydrochalcones aspalathin and nothofagin and their corresponding flavones in relation to other rooibos (Aspalathus linearis) flavonoids, epigallocatechin gallate, and Trolox. J. Agric. Food Chem. 2009, 57, 6678–6684. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive com-pounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT-Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Koteswara, C.; Sung, E.; Young, S.; Hwan, C. Inclusion complexation of catechins-rich green tea extract by β-cyclodextrin: Preparation, physicochemical, thermal, and antioxidant properties. LWT—Food Sci. Technol. 2020, 131, 1–10. [Google Scholar]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crop. Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]







| Run | Extraction Conditions | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | Y5 | |
| β-CD Concentrations | Extraction Temperature | Treatment Time | Total Polyphenolic Content (TPC) | Iron Chelation (MTC) | Radical Scavenging (ABTS) | Reducing Power (FRAP) | Total Antioxidant Activity (ORAC) | |
| (mM) | (°C) | (min) | (mg GAE·g−1) | % | (µmol TE·g−1) | (µmol AAE·g−1) | (µmol TE·g−1) | |
| 1 | (+1)15 | (1)90 | (−1)15 | 374.73 ± 30.28 bcd | 70.99 ± 2.51 de | 1425.31 ± 69.58 bc | 1886.42 ± 138.72 c | 11,791.39 ± 422.51 d |
| 2 | (−1)0 | (0)65 | (1)60 | 299.5 ± 23.17 a | 44.0 ± 8.54 ab | 1092.0 ± 29.80 a | 1576.50 ± 3.08 a | 7574.93 ± 119.89 a |
| 3 | (+1)15 | (−1)40 | (1)60 | 398.25 ± 15.97 d | 92.95 ± 17.87 f | 1689.70 ± 23.88 d | 2097.53 ± 22.33 d | 11,162.82 ± 104.32 cd |
| 4 | (−1)0 | (1)90 | (0)30 | 281.7 ± 2.36 a | 38.00 ± 5.57 a | 1056.86 ± 62.84 a | 1472.78 ± 39.48 a | 6948.02 ± 391.44 a |
| 5 | (0)7.5 | (0)90 | (1)60 | 361.84 ± 23.80 bc | 52.82 ± 3.20 abc | 1351.52 ± 14.79 b | 1830.63 ± 63.83 bc | 10,813.97 ± 123.11 c |
| 6 | (+1)15 | (0)65 | (0)30 | 387.81 ± 14.26 cd | 84.57 ± 13.01 ef | 1476.82 ± 89.61 c | 1927.37 ± 46.28 c | 11,334.79 ± 270.66 cd |
| 7 | (0)7.5 | (0)65 | (−1)15 | 351.62 ± 20.62 b | 59.09 ± 2.62 bcd | 1346.96 ± 58.07 b | 1863.14 ± 140.69 bc | 9521.48 ± 356.62 b |
| 8 | (0)7.5 | (−1)40 | (0)30 | 344.00 ± 2.48 b | 61.03 ± 2.93 cd | 1351.61 ± 28.58 b | 1732.12 ± 11.09 b | 8903.31 ± 279.79 b |
| 9 | (−1)0 | (−1)40 | (−1)15 | 305.5 ± 21.00 a | 41.00 ± 9.64 a | 1050.70 ± 61.64 a | 1574.80 ± 69.01 a | 7234.37 ± 14.59 a |
| Run | β-CD (mM) | Temp (°C) | Time (min) | Aspalathin (mg·g−1) | Isorientin (mg·g−1) | Orientin (mg·g−1) | Isoviten (mg·g−1) | Vitexin (mg·g−1) | Hyperoside (mg·g−1) | Quercetin (mg·g−1) | Luteolin (mg·g−1) | Chrysoeriol (mg·g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 90 | 15 | 136.32 ± 11.64 b | 5.87 ± 0.33 b | 10.03 ± 1.78 a | 1.73 ± 0.63 ab | 2.34 ± 0.18 ab | 23.34 ± 4.87 a | 0.110 ± 0.01 c | 0.143 ± 0.04 c | 0.063 ± 0.01 c |
| 2 | 0 | 65 | 60 | 93.93 ±15.25 a | 5.94 ± 0.90 b | 8.88 ± 0.37 a | 1.68 ± 0.45 ab | 1.95 ± 0.16 bc | 21.34 ± 3.43 a | 0.090 ± 0.02 bc | 0.043 ± 0.01 a | 0.043 ± 0.02 ab |
| 3 | 15 | 40 | 60 | 172.25 ± 7.61 c | 7.93 ± 0.21 c | 8.86 ± 1.17 a | 2.31 ± 0.14 c | 2.53 ± 0.20 e | 29.27 ± 1.46 c | 0.137 ± 0.01 d | 0.070 ± 0.01 ab | 0.060 ± 0.00 c |
| 4 | 0 | 90 | 30 | 96.20 ± 4.22 a | 4.44 ± 0.56 a | 9.97 ± 1.16 a | 1.89 ± 0.07 abc | 1.76 ± 0.10 ab | 23.12 ± 0.56 ab | 0.080 ± 0.00 bc | 0.077 ± 0.01 b | 0.040 ± 0.00 ab |
| 5 | 7.5 | 90 | 60 | 107.70 ± 3.63 a | 4.14 ± 1.27 a | 10.17± 0.43 a | 1.55 ± 0.19 a | 1.69 ± 0.10 a | 19.61 ± 2.28 a | 0.070 ± 0.01 b | 0.057 ± 0.02 ab | 0.030 ± 0.01 a |
| 6 | 15 | 65 | 30 | 148.07± 11.10 b | 6.56 ± 0.23 b | 9.51 ± 0.99 a | 1.77 ± 0.38 abc | 2.49 ± 0.14 e | 23.55 ± 4.27 bc | 0.063 ± 0.02 b | 0.083± 0.01 b | 0.050 ± 0.00 bc |
| 7 | 7.5 | 65 | 15 | 134.48 ± 12.04 b | 5.51 ± 0.31 b | 10.60 ± 1.06 a | 1.37 ± 0.76 a | 2.30 ± 0.14 de | 21.26 ± 1.02 a | 0.040 ± 0.00 a | 0.063 ± 0.00 ab | 0.043 ± 0.00 ab |
| 8 | 7.5 | 40 | 30 | 143.23 ± 6.96 b | 4.05 ± 0.16 a | 13.46 ± 1.34 b | 2.24 ± 0.14 bc | 2.34 ± 0.17 de | 27.53 ± 1.15 bc | 0.107 ± 0.02 c | 0.083 ± 0.00 b | 0.050 ± 0.01 bc |
| 9 | 0 | 40 | 15 | 93.52 ± 3.58 a | 5.98 ± 0.18 b | 10.12 ± 0.36 a | 1.42 ± 0.04 a | 2.16 ± 0.08 cd | 18.75 ± 0.20 a | 0.083 ± 0.01 bc | 0.053 ± 0.01 bc | 0.043 ± 0.02 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. https://doi.org/10.3390/molecules27113556
Vhangani LN, Favre LC, Rolandelli G, Van Wyk J, del Pilar Buera M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules. 2022; 27(11):3556. https://doi.org/10.3390/molecules27113556
Chicago/Turabian StyleVhangani, Lusani Norah, Leonardo Cristian Favre, Guido Rolandelli, Jessy Van Wyk, and María del Pilar Buera. 2022. "Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction" Molecules 27, no. 11: 3556. https://doi.org/10.3390/molecules27113556