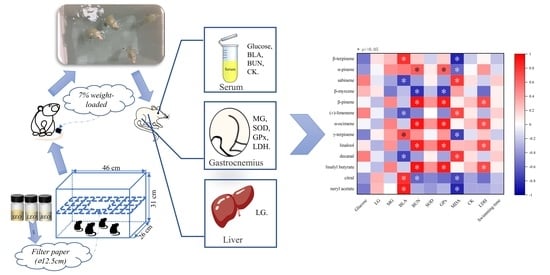
A Comparative Study on Relieving Exercise-Induced Fatigue by Inhalation of Different Citrus Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the CEOs
2.2. Effect of the Inhalation of CEOs on Physiological Indices in Rats
2.3. Effect of the Inhalation of CEOs on Exercise Performance in Rats
2.4. Effect of the Inhalation of CEOs on Energy Supply in Rats
2.5. Effect of the Inhalation of CEOs on Metabolite Accumulation in Rats
2.6. Effect of the Inhalation of CEOs on Oxidative Stress in Rats
2.7. Effect of the Inhalation of CEOs on Muscle Injury in Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. GC-MS
4.3. Experimental Animals
4.4. Animal Experiment Design
4.5. Exhaustive Swimming Test
4.6. Determination of Fatigue-Associated Biochemical Parameters
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| ROS | reactive oxygen species |
| EOs | essential oils |
| CEOs | citrus essential oils |
| SEO | sweet orange essential oil |
| LEO | lemon essential oil |
| BEO | bergamot essential oil |
| GC-MS | gas chromatography-mass spectrometer |
| CON | control |
| FC | fatigue control |
| BLA | blood lactic acid |
| BUN | blood urea nitrogen |
| CK | creatine kinase |
| LG | liver glycogen |
| MG | muscle glycogen |
| LDH | lactate dehydrogenase |
| GSH-PX | glutathione peroxidase |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| RT | retention time |
| ND | not detected |
References
- Wang, P.; Wang, D.; Hu, J.; Tan, B.K.; Zhang, Y.; Lin, S. Natural Bioactive Peptides to Beat Exercise-Induced Fatigue: A Review. Food Biosci. 2021, 43, 101298. [Google Scholar] [CrossRef]
- Surhio, M.M.; Wang, Y.; Fang, S.; Li, J.; Ye, M. Anti-Fatigue Activity of a Lachnum Polysaccharide and Its Carboxymethylated Derivative in Mice. Bioorganic Med. Chem. Lett. 2017, 27, 4777–4780. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ren, J.W.; Zhang, T.; Liu, R.; Wu, L.; Du, Q.; Li, Y. Anti-Fatigue Effects of Small-Molecule Oligopeptides Isolated from Panax quinquefolium L. in Mice. Food Funct. 2018, 9, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Xing, H.; Tian, B.; Xu, J.; Li, Z.; Zhu, H.; Yang, K.; Sun, P. Characteristics and Antifatigue Activity of Graded Polysaccharides from Ganoderma Lucidum Separated by Cascade Membrane Technology. Carbohydr. Polym. 2021, 269, 118329. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Lee, S.J.; Adam, G.O.; Kim, S.J. Aralia Continentalis Kitagawa Extract Attenuates the Fatigue Induced by Exhaustive Exercise through Inhibition of Oxidative Stress. Antioxidants 2020, 9, 379. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.W.; Chen, Q.H.; Li, D.; Mao, R.X.; Liu, X.R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2019, 24, 45. [Google Scholar] [CrossRef] [Green Version]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-Limonene Exhibits Anti-Inflammatory and Antioxidant Properties in an Ulcerative Colitis Rat Model via Regulation of INOS, COX-2, PGE2 and ERK Signaling Pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef] [Green Version]
- Scuteri, D.; Rombolà, L.; Morrone, L.A.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Tonin, P.; Corasaniti, M.T. Neuropharmacology of the Neuropsychiatric Symptoms of Dementia and Role of Pain: Essential Oil of Bergamot as a Novel Therapeutic Approach. Int. J. Mol. Sci. 2019, 20, 3327. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.C.; Wang, S.H.; Huang, C.C.; Lai, Y.C.; Song, T.Y.; Tsai, M.S. Anti-Fatigue, Antioxidation, and Anti- Inflammatory Effects of Eucalyptus Oil Aromatherapy in Swimming-Exercised Rats. Chin. J. Physiol. 2018, 61, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, F.; Shao, H.; Zhang, Y.; Fan, A.; Li, F. Does the Fragrance of Essential Oils Alleviate the Fatigue Induced by Exercise? A Biochemical Indicator Test in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 5027372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campêlo, L.M.L.; Gonçalves, F.C.M.; Feitosa, C.M.; de Freitas, R.M. Antioxidant Activity of Citrus Limon Essential Oil in Mouse Hippocampus. Pharm. Biol. 2011, 49, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goes, T.C.; Antunes, F.D.; Alves, P.B.; Teixeira-Silva, F. Effect of Sweet Orange Aroma on Experimental Anxiety in Humans. J. Altern. Complement. Med. 2012, 18, 798–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. General Safety Guidelines. In Essential Oil Safety, 2nd ed.; Tisserand, R., Young, R., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2014; pp. 649–654. ISBN 978-0-443-06241-4. [Google Scholar]
- Bai, J.; Zheng, Y.; Wang, G.; Liu, P. Protective Effect of D-Limonene against Oxidative Stress-Induced Cell Damage in Human Lens Epithelial Cells via the P38 Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 5962832. [Google Scholar] [CrossRef]
- Cui, Y.; Che, Y.; Wang, H. Bergamot Essential Oil Attenuate Aluminum-Induced Anxiety-like Behavior through Antioxidation, Anti-Inflammatory and GABA Regulation in Rats. Food Chem. Toxicol. 2020, 145, 111766. [Google Scholar] [CrossRef]
- De Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of Sweet Orange Essential Oil (Citrus aurantium Var. Dulcis) by Liophylization Using Maltodextrin and Maltodextrin/Gelatin Mixtures: Preparation, Characterization, Antimicrobial and Antioxidant Activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef]
- Nagai, K.; Horii, Y.; Fujisaki, Y.; Fuyuki, R.; Misonou, Y. Effects of Olfactory Stimulation with Scents of Grapefruit and Lavender Essential Oils on the Skeletal Muscle Sympathetic Nerve and Muscle Blood Flow in Rats. Flavour Fragr. J. 2018, 33, 135–143. [Google Scholar] [CrossRef]
- Gioffrè, G.; Ursino, D.; Concetta Labate, M.L.; Giuffrè, A.M. The Peel Essential Oil Composition of Bergamot Fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A Review. Emir. J. Food Agric. 2020, 32, 835–845. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and Bioactives in Citrus Fruits: Different Citrus Varieties, Fruit Parts, and Growth Stages. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. Mechanisms of Physical Fatigue and Its Applications in Nutritional Interventions. J. Agric. Food Chem. 2021, 69, 6755–6768. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Lyu, C.; Li, Q.; Kou, F.; Jiang, M.; Wei, H. Metabolomics Study on the Intervention Effect of Radix Salviae Miltiorrhizae Extract in Exercise-Induced Exhaustion Rat Using Gas Chromatography Coupled to Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1178, 122805. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, F.; Wu, Q.; Luo, Y.; Guo, T.; Han, S.; Huang, M.; Hu, Z.; Bai, J.; Luo, F.; et al. Dietary Supplementation of Octacosanol Improves Exercise-Induced Fatigue and Its Molecular Mechanism. J. Agric. Food Chem. 2021, 69, 7603–7618. [Google Scholar] [CrossRef]
- Zhang, Y.; Ryu, B.; Cui, Y.; Li, C.; Zhou, C.; Hong, P.; Lee, B.; Qian, Z.-J. A Peptide Isolated from Hippocampus Abdominalis Improves Exercise Performance and Exerts Anti-Fatigue Effects via AMPK/PGC-1α Pathway in Mice. J. Funct. Foods 2019, 61, 103489. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Yadav, M.P. Insights into the Chemical Composition and Bioactivities of Citrus Peel Essential Oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef]
- Niijima, A.; Nagai, K. Effect of Olfactory Stimulation with Flavor of Grapefruit Oil and Lemon Oil on the Activity of Sympathetic Branch in the White Adipose Tissue of the Epididymis. Exp. Biol. Med. 2003, 228, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, H.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. 18Β-Glycyrrhetinic Acid Improves High-Intensity Exercise Performance by Promoting Glucose-Dependent Energy Production and Inhibiting Oxidative Stress in Mice. Phytother. Res. 2021, 35, 6932–6943. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, J.; Zhong, H.; Abdullah; Zhuang, J.; Zhang, J.; Wang, J.; Zhang, X.; Feng, F. High-Degree Hydrolysis Sea Cucumber Peptides Improve Exercise Performance and Exert Antifatigue Effect via Activating the NRF2 and AMPK Signaling Pathways in Mice. J. Funct. Foods 2021, 86, 104677. [Google Scholar] [CrossRef]
- Gonzalez-Rellan, M.J.; Fondevila, M.F.; Fernandez, U.; Rodríguez, A.; Varela-Rey, M.; Veyrat-Durebex, C.; Seoane, S.; Bernardo, G.; Lopitz-Otsoa, F.; Fernández-Ramos, D.; et al. O-GlcNAcylated P53 in the Liver Modulates Hepatic Glucose Production. Nat. Commun. 2021, 12, 1–21. [Google Scholar] [CrossRef]
- Adams, O.P. The Impact of Brief High-Intensity Exercise on Blood Glucose Levels. Diabetes Metab. Syndr. Obes. 2013, 6, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.-H.; Ker, Y.-B.; Weng, C.-F.; Peng, C.-C.; Huang, C.-N.; Lin, L.-Y.; Peng, R.Y. Insulin Secretagogue Bioactivity of Finger Citron Fruit (Citrus medica L. Var. Sarcodactylis hort, Rutaceae). J. Agric. Food Chem. 2009, 57, 8812–8819. [Google Scholar] [CrossRef] [PubMed]
- Marliss, E.B.; Vranic, M. Intense Exercise Has Unique Effects on Both Insulin Release and Its Roles in Glucoregulation: Implications for diabetes. Diabetes 2002, 51, S271–S283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, P.; Suh, S.H.; Min, S.S.; Seol, G.H. The Essential Oil of Citrus Bergamia Risso Induces Vasorelaxation of the Mouse Aorta by Activating K+ Channels and Inhibiting Ca2+ Influx. J. Pharm. Pharmacol. 2013, 65, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential Oil of Citrus Lumia Risso: Phytochemical Profile, Antioxidant Properties and Activity on the Central Nervous System. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. A.T Peana–Copy. Phytomedicine 2002, 9, 721–726. [Google Scholar]
- Muhammad, I. Antioxidant and Antimicrobial Activities of Essential Oil of Skimmea Laureola Growing Wild in the State of Jammu and Kashmir. J. Med. Plants Res. 2012, 6, 1680–1684. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus Spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [Green Version]
- Yip, Y.B.; Tam, A.C.Y. An Experimental Study on the Effectiveness of Massage with Aromatic Ginger and Orange Essential Oil for Moderate-to-Severe Knee Pain among the Elderly in Hong Kong. Complement. Ther. Med. 2008, 16, 131–138. [Google Scholar] [CrossRef]
- Ikeda, H.; Takasu, S.; Murase, K. Contribution of Anterior Cingulate Cortex and Descending Pain Inhibitory System to analgesic Effect of Lemon Odor in Mice. Mol. Pain 2014, 10, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, Y.N.; Xu, M.; Ren, J.N.; Dong, M.; Yang, Z.Y.; Pan, S.Y.; Fan, G. Optimisation of α-Terpineol Production by Limonene Biotransformation Using Penicillium Digitatum DSM 62840. J. Sci. Food Agric. 2016, 96, 954–961. [Google Scholar] [CrossRef] [PubMed]








| No. | RT/(min) | Compound | Molecular Formula | Relative Content (%) | ||
|---|---|---|---|---|---|---|
| SEO | LEO | BEO | ||||
| 1 | 10.52 | β-terpinene | C10H16 | 2.18 | 11.56 | 9.95 |
| 2 | 11.32 | tricyclene | C10H16 | ND | ND | 0.15 |
| 3 | 11.93 | α-pinene | C10H16 | 0.03 | 3.57 | 5.10 |
| 4 | 12.06 | β-phellandrene | C10H16 | 0.02 | ND | ND |
| 5 | 12.60 | sabinene | C10H16 | 1.76 | ND | ND |
| 6 | 12.62 | β-thujene | C10H16 | ND | 0.11 | ND |
| 7 | 13.33 | p-cymene | C10H14 | ND | ND | 0.01 |
| 8 | 13.72 | β-myrcene | C10H16 | 4.62 | 2.51 | ND |
| 9 | 13.77 | β-pinene | C10H16 | 0.09 | ND | 4.62 |
| 10 | 14.05 | sylvestrene | C10H16 | ND | ND | 0.61 |
| 11 | 15.85 | (±)-limonene | C10H16 | 81.65 | 38.63 | 32.92 |
| 12 | 16.69 | α-ocimene | C10H16 | ND | ND | 2.94 |
| 13 | 16.73 | (+)-3-carene | C10H16 | 0.21 | 0.35 | ND |
| 14 | 17.17 | γ-terpinene | C10H16 | 1.20 | 7.68 | 8.15 |
| 15 | 17.51 | trans-isolimonene | C10H16 | ND | ND | 0.08 |
| 16 | 18.42 | α-terpinolene | C10H16 | ND | 0.45 | 0.91 |
| 17 | 18.44 | 2-carene | C10H16 | 0.11 | ND | ND |
| 18 | 19.03 | sabinene hydrate | C10H18O | ND | 0.04 | ND |
| 19 | 19.43 | nonanal | C9H18O | 0.14 | ND | ND |
| 20 | 19.47 | linalool | C10H18O | 2.77 | 0.89 | 7.67 |
| 21 | 19.48 | 1-cyclopropylpentane | C8H16 | ND | 0.08 | ND |
| 22 | 21.10 | (+)-2-bornanone | C10H16O | ND | ND | 0.05 |
| 23 | 21.19 | 1,3,8-p-menthatriene | C10H14 | ND | 0.08 | ND |
| 24 | 21.30 | β-fenchol | C10H18O | ND | 0.04 | ND |
| 25 | 21.70 | trans-p-mentha-2,8-dienol | C10H16O | ND | 0.08 | ND |
| 26 | 22.20 | neo-allo-ocimene | C10H16 | ND | 0.01 | 0.28 |
| 27 | 22.28 | limonene 1,2-epoxide | C10H16O | 0.03 | 0.05 | 0.02 |
| 28 | 23.42 | citronellal | C10H18O | 0.09 | 0.19 | ND |
| 29 | 22.44 | 1R,4R-p-mentha-2,8-dien-1-ol | C10H16O | ND | 0.20 | ND |
| 30 | 23.66 | pinocarvone | C10H14O | ND | 0.04 | ND |
| 31 | 24.46 | (−)-terpinen-4-ol | C10H18O | ND | 0.12 | ND |
| 32 | 24.43 | decanal | C10H20O | 2.53 | ND | ND |
| 33 | 24.71 | cyclooctane | C8H16 | 0.02 | ND | ND |
| 34 | 25.20 | α-terpineol | C10H18O | ND | 0.35 | ND |
| 35 | 26.04 | cis-citral | C10H16O | 0.48 | 6.41 | 0.13 |
| 36 | 26.47 | linalyl butyrate | C14H24O2 | ND | ND | 17.83 |
| 37 | 27.39 | trans-citral | C10H16O | 0.87 | ND | 0.06 |
| 38 | 27.48 | cis-carveol | C10H16O | ND | 0.20 | ND |
| 39 | 28.25 | 3-cyclohexen-1-one, 2-isopropyl-5-methyl- | C10H16O | ND | 0.06 | ND |
| 40 | 29.60 | citral | C10H16O | ND | 12.09 | ND |
| 41 | 30.84 | α-terpinyl acetate | C12H20O2 | ND | ND | 0.05 |
| 42 | 31.67 | neryl acetate | C12H20O2 | 0.07 | 4.17 | 1.48 |
| 43 | 32.55 | geranyl acetate | C12H20O2 | ND | 4.05 | 2.24 |
| 44 | 32.48 | bicyclo [4.4.0] dec-1-ene, 2-isopropyl-5-methyl-9-methylene- | C15H24 | 0.10 | ND | ND |
| 45 | 32.59 | beta-elemene | C15H24 | 0.04 | ND | ND |
| 46 | 33.47 | dodecanal | C12H24O | 0.02 | ND | ND |
| 47 | 33.70 | caryophyllene | C15H24 | 0.13 | 1.32 | 0.34 |
| 48 | 34.09 | α-cubebene | C15H24 | 0.14 | ND | ND |
| 49 | 34.46 | α-bergamotene | C15H24 | ND | 0.07 | 0.11 |
| 50 | 36.77 | (+)-valencene | C15H24 | 0.38 | ND | ND |
| 51 | 38.04 | δ-cadinene | C15H24 | 0.06 | ND | ND |
| 52 | 39.18 | β-bisabolene | C15H24 | ND | 0.09 | 0.04 |
| Groups | Organ Indices (%) | ||
|---|---|---|---|
| Liver Indices | Spleen Indices | Kidney Indices | |
| CON | 3.85 ± 0.34 a | 0.16 ± 0.02 a | 0.67 ± 0.06 b |
| FC | 3.56 ± 0.20 ab | 0.17 ± 0.02 a | 0.65 ± 0.03 b |
| SEO | 3.40 ± 0.28 b | 0.17 ± 0.03 a | 0.74 ± 0.03 a |
| LEO | 3.43 ± 0.40 b | 0.15 ± 0.01 a | 0.74 ± 0.04 a |
| BEO | 3.37 ± 0.25 b | 0.16 ± 0.02 a | 0.74 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Hu, T.; Zhang, S.; Zhang, H.; Yang, C.; Chen, G.; Pan, S. A Comparative Study on Relieving Exercise-Induced Fatigue by Inhalation of Different Citrus Essential Oils. Molecules 2022, 27, 3239. https://doi.org/10.3390/molecules27103239
Tian L, Hu T, Zhang S, Zhang H, Yang C, Chen G, Pan S. A Comparative Study on Relieving Exercise-Induced Fatigue by Inhalation of Different Citrus Essential Oils. Molecules. 2022; 27(10):3239. https://doi.org/10.3390/molecules27103239
Chicago/Turabian StyleTian, Lei, Tan Hu, Shanshan Zhang, Hongyan Zhang, Chenxi Yang, Guiting Chen, and Siyi Pan. 2022. "A Comparative Study on Relieving Exercise-Induced Fatigue by Inhalation of Different Citrus Essential Oils" Molecules 27, no. 10: 3239. https://doi.org/10.3390/molecules27103239