Manipulation of the Phytochemical Profile of Tenderstem® Broccoli Florets by Short Duration, Pre-Harvest LED Lighting
Abstract
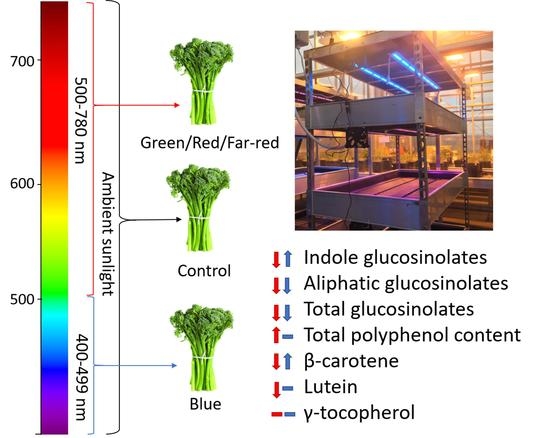
:1. Introduction
2. Results
2.1. Effect of Light Treatment on Tenderstem® Morphology
2.2. Effect of Light Treatments on Phytochemical Content of Broccoli
2.2.1. Total Polyphenols
2.2.2. Glucosinolate Content
2.2.3. Carotenoids and γ-Tocopherol
3. Discussion
4. Materials and Methods
4.1. Plant Experimental Set-Up
4.1.1. Plant Growth before Treatment
4.1.2. Light Treatments
4.1.3. Timing of Treatments
4.1.4. Floret Samples
4.2. Determination of Total Polyphenol Content
4.3. Determination of GLS Content
4.4. Determination of Carotenoids and Tocopherol
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in Broccoli: The Green Chemoprevention!! Role in Cancer Prevention and Therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M. Vegetable Crop Management Strategies to Increase the Quantity of Phytochemicals. Eur. J. Nutr. 2005, 44, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Villani, A.; Paciolla, F.; Mul, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The Evaluation of Growth Performance, Photosynthetic Capacity, and Primary and Secondary Metabolite Content of Leaf Lettuce Grown under Limited Irradiation of Blue and Red LED Light in an Urban Plant Factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.J.; Zhang, Y.T.; Liu, H.C.; Li, Y.M.; Liu, Y.L.; Hao, Y.W.; Lei, B.F. Supplemental Blue Light Increases Growth and Quality of Greenhouse Pak Choi Depending on Cultivar and Supplemental Light Intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef] [Green Version]
- Brazaityte, A.; Sakalauskiene, S.; Samuoliene, G.; Jankauskiene, J.; Viršile, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The Effects of LED Illumination Spectra and Intensity on Carotenoid Content in Brassicaceae Microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. LED-Induced Carotenoid Synthesis and Related Gene Expression in Brassica Microgreens. J. Agric. Food Chem. 2021, 69, 4674–4685. [Google Scholar] [CrossRef]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Beatriz, M.; Oliveira, P.P. Evaluating the Impact of Sprouting Conditions on the Glucosinolate Content of Brassica oleracea Sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-Band Light-Emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in Shoot Tissue Pigments, Glucosinolates, and Mineral Elements in Sprouting Broccoli after Exposure to Short-Duration Blue Light from Light Emitting Diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light Intensity and Quality from Sole-Source Light-Emitting Diodes Impact Growth, Morphology, and Nutrient Content of Brassica Microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential Effects of Low Light Intensity on Broccoli Microgreens Growth and Phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Jankauskiene, J.; Viršile, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED Irradiance Level Affects Growth and Nutritional Quality of Brassica Microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Johansen, T.J.; Bengtsson, G.B.; Hagen, S.F.; Mølmann, J.A.B. Impact of Pre-Harvest Light Spectral Properties on Health- and Sensory-Related Compounds in Broccoli Florets. J. Sci. Food Agric. 2016, 96, 1974–1981. [Google Scholar] [CrossRef]
- Loi, M.; Liuzzi, V.C.; Fanelli, F.; De Leonardis, S.; Maria Creanza, T.; Ancona, N.; Paciolla, C.; Mulè, G. Effect of Different Light-Emitting Diode (LED) Irradiation on the Shelf Life and Phytonutrient Content of Broccoli (Brassica oleracea L. Var. Italica). Food Chem. 2019, 283, 206–214. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Zamora, L.; Gómez, P.A.; Pennisi, G.; Crepaldi, A.; Fernández, J.A.; Orsini, F.; Artés-Hernández, F. Postharvest LED Lighting: Effect of Red, Blue and Far Red on Quality of Minimally Processed Broccoli Sprouts. J. Sci. Food Agric. 2021, 101, 44–53. [Google Scholar] [CrossRef]
- Son, K.H.; Park, J.H.; Kim, D.; Oh, M.M. Leaf Shape Index, Growth, and Phytochemicals in Two Leaf Lettuce Cultivars Grown under Monochromatic Light-Emitting Diodes. Korean J. Hortic. Sci. Technol. 2013, 31, 664–672. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of Phenolic Acid and Flavonoid Synthesis to Blue and Blue-Violet Light Depends on Plant Species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Mølmann, J.A.B.; Steindal, A.L.H.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of Temperature and Photoperiod on Sensory Quality and Contents of Glucosinolates, Flavonols and Vitamin C in Broccoli Florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef]
- Steindal, A.L.H.; Mølmann, J.; Bengtsson, G.B.; Johansen, T.J. Influence of Day Length and Temperature on the Content of Health-Related Compounds in Broccoli (Brassica oleracea L. Var. Italica). J. Agric. Food Chem. 2013, 61, 10779–10786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente Pereira, F.M.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of Temperature and Ontogeny on the Levels of Glucosinolates in Broccoli (Brassica oleracea Var. Italica) Sprouts and Their Effect on the Induction of Mammalian Phase 2 Enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef] [PubMed]
- Mitreiter, S.; Gigolashvili, T. Regulation of Glucosinolate Biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid Metabolism and Regulation in Horticultural Crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santin, M.; Ranieri, A.; Castagna, A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Renaud, E.N.C.; Lammerts Van Bueren, E.T.; Myers, J.R.; Paulo, M.J.; Van Eeuwijk, F.A.; Zhu, N.; Juvik, J.A. Variation in Broccoli Cultivar Phytochemical Content under Organic and Conventional Management Systems: Implications in Breeding for Nutrition. PLoS ONE 2014, 9, e95683. [Google Scholar] [CrossRef] [Green Version]
- Cela, J.; Chang, C.; Munné-Bosch, S. Accumulation of γ-Rather than α-Tocopherol Alters Ethylene Signaling Gene Expression in the Vte4 Mutant of Arabidopsis Thaliana. Plant Cell Physiol. 2011, 52, 1389–1400. [Google Scholar] [CrossRef] [Green Version]
- Wurr, D.C.E.; Hambidge, A.J.; Fellows, J.R.; Lynn, J.R.; Pink, D.A.C. The Influence of Water Stress during Crop Growth on the Postharvest Quality of Broccoli. Postharvest Biol. Technol. 2002, 25, 193–198. [Google Scholar] [CrossRef]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.A.; Rossiter, J.T.; Mithen, R.F.; Kroon, P.A. Isothiocyanate Concentrations and Interconversion of Sulforaphane to Erucin in Human Subjects after Consumption of Commercial Frozen Broccoli Compared to Fresh Broccoli. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef]





| Trial 1 | Trial 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harvest 1 | Harvest 2 | Harvest 1 | Harvest 2 | |||||||||
| M | SD | SE | M | SD | SE | M | SD | SE | M | SD | SE | |
| Light treatment | (Fresh weight g) | |||||||||||
| Co | 110.23 | 20.45 | 4.57 | 71.45 | 13.38 | 2.99 | 53.58 | 21.73 | 4.86 | 53.22 | 11.16 | 2.50 |
| B | 84.53 | 19.29 | 4.31 | 59.78 | 14.48 | 3.24 | 33.74 | 19.67 | 4.40 | 39.22 | 18.46 | 4.18 |
| G-R-FR | 89.09 | 19.88 | 4.44 | 68.73 | 19.21 | 4.30 | 45.32 | 15.37 | 3.44 | 63.81 | 21.77 | 4.87 |
| Trial 1 | Trial 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Harvest 1 | Harvest 2 | Harvest 1 | Harvest 2 | |||||||||
| M | SD | SE | M | SD | SE | M | SD | SE | M | SD | SE | |
| Light treatments | (Height to spike cm) | |||||||||||
| Co | 72.60 | 6.77 4.17 | 1.51 | 95.75 | 9.62 0.00 | 2.15 | 80.55 | 5.16 4.29 | 1.15 | 114.75 | 10.45 0.00 | 2.34 |
| B | 70.25 | 4.17 | 0.93 | 80.00 | 0.00 | 0.00 | 77.95 | 4.29 | 0.96 | 80.00 | 0.00 | 0.00 |
| G-R-FR | 70.75 | 3.13 | 0.70 | 80.00 | 0.00 | 0.00 | 78.75 | 2.22 | 0.50 | 80.00 | 0.00 | 0.00 |
| Light Source | Distribution (%) | ||||
|---|---|---|---|---|---|
| 380–399 nm UV | 400–499 nm Blue | 500–599 nm Green | 600–700 nm Red | 701–780 nm Far-Red | |
| B LED | 0 | 99 | 1 | 0 | 0 |
| G-R-FR LED | 0 | 2 | 11 | 62 | 25 |
| Co LED | 0 | 7 | 11 | 81 | 1 |
| Sunlight | 0 | 19 | 28 | 30 | 23 |
| Light Source | Irradiance (μmol m–2 s–1) | |
|---|---|---|
| PFD 380–780 nm | PPFD 400–700 nm | |
| B LED | 160 | 160 |
| G-R-FR LED | 213 | 160 |
| Co LED | 95 | 94 |
| Sunlight | 675 | 517 |
| Trial | Light Treatment | Growth Period | Temperature °C | Relative Humidity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Average | Max | Min | Average | Max | Min | |||
| 1 | G-R-FR | Week 11–13 | 22.5 | 43.8 | 10.9 | 78.4 | 100.0 | 14.7 |
| B | Week 11–13 | 20.2 | 32.5 | 11.0 | 79.1 | 100.0 | 41.1 | |
| Co | Week 1–10 | 17.5 | 39.8 | 4.3 | 64.1 | 100.0 | 22.2 | |
| Co | Week 11–13 | 20.0 | 36.2 | 9.3 | 75.9 | 100.0 | 38.4 | |
| 2 | G-R-FR | Week 11–13 | 25.2 | 40.8 | 16.3 | 72.3 | 91.6 | 44.2 |
| B | Week 11–13 | 24.6 | 37.3 | 16.6 | 74.0 | 91.4 | 49.6 | |
| Co | Week 1–10 | 22.0 | 45.8 | 5.4 | 67.7 | 100.0 | 11.6 | |
| Co | Week 11–13 | 24.5 | 41.0 | 16.1 | 61.7 | 83.5 | 25.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langston, F.M.A.; Monaghan, J.M.; Cousins, O.; Nash, G.R.; Bows, J.R.; Chope, G. Manipulation of the Phytochemical Profile of Tenderstem® Broccoli Florets by Short Duration, Pre-Harvest LED Lighting. Molecules 2022, 27, 3224. https://doi.org/10.3390/molecules27103224
Langston FMA, Monaghan JM, Cousins O, Nash GR, Bows JR, Chope G. Manipulation of the Phytochemical Profile of Tenderstem® Broccoli Florets by Short Duration, Pre-Harvest LED Lighting. Molecules. 2022; 27(10):3224. https://doi.org/10.3390/molecules27103224
Chicago/Turabian StyleLangston, Faye M. A., James M. Monaghan, Olivia Cousins, Geoffrey R. Nash, John R. Bows, and Gemma Chope. 2022. "Manipulation of the Phytochemical Profile of Tenderstem® Broccoli Florets by Short Duration, Pre-Harvest LED Lighting" Molecules 27, no. 10: 3224. https://doi.org/10.3390/molecules27103224