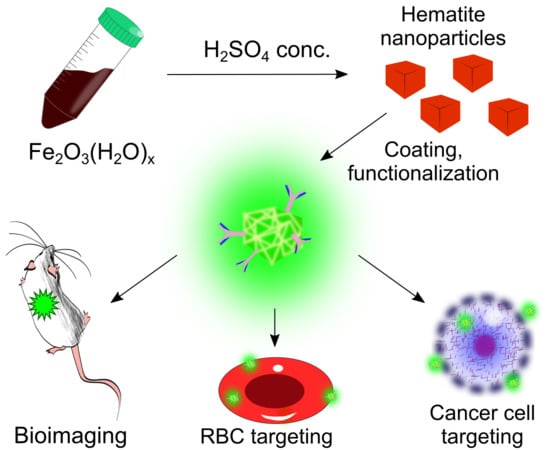
Hematite Nanoparticles from Unexpected Reaction of Ferrihydrite with Concentrated Acids for Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwertmann, U.; Cornell, M.R. Iron Oxides in the Laboratory; Schwertmann, U., Cornell, R.M., Eds.; Wiley: Weinheim, Germany, 2000; Volume 104. [Google Scholar]
- Dehouck, E.; McLennan, S.M.; Sklute, E.C.; Dyar, M.D. Stability and fate of ferrihydrite during episodes of water/rock interactions on early Mars: An experimental approach. J. Geophys. Res. Planets 2017, 122, 358–382. [Google Scholar] [CrossRef]
- Sill, G.T. Chemical Eactions at the Surface and in the Atmosphere of Venus; American Geophysical Union: Washington, DC, USA, 1976. [Google Scholar]
- Schwertmann, U.; Murad, E. Effect of pH on the formation of goethite and hematite from ferrihydrite. Clays Clay Miner. 1983, 31, 277–284. [Google Scholar] [CrossRef]
- Schwertmann, U.; Friedl, J.; Stanjek, H. From Fe(III) ions to ferrihydrite and then to hematite. J. Colloid Interface Sci. 1999, 209, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Stanjek, H.; Becher, H.-H. Long-term in vitro transformation of 2-line ferrihydrite to goethite/hematite at 4, 10, 15 and 25 °C. Clay Miner. 2004, 39, 433–438. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Kim, C.S.; Banfield, J.F. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms. J. Nanoparticle Res. 2005, 7, 409–433. [Google Scholar] [CrossRef]
- Burleson, D.J.; Penn, R.L. Two-step growth of goethite from ferrihydrite. Langmuir 2006, 22, 402–409. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of two-line ferrihydrite to goethite and hematite as a function of pH and temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Dekkers, M.J.; Barron, V.; Torrent, J.; Roberts, A.P. Control of Earth-like magnetic fields on the transformation of ferrihydrite to hematite and goethite. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Raming, T.P.; Winnubst, A.J.A.; Van Kats, C.M.; Philipse, A.P. The synthesis and magnetic properties of nanosized hematite (α-Fe2O3) particles. J. Colloid Interface Sci. 2002, 249, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.B.; Min, Y.L.; Yu, S.H. Synthesis and magnetic properties of uniform hematite nanocubes. J. Phys. Chem. C 2007, 111, 3551–3554. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Surya, R.; Filser, S.; Wimmer, A.; Weigl, F.; Fraga-García, P.; Berensmeier, S. Formation of iron oxide nanoparticles for the photooxidation of water: Alteration of finite size effects from ferrihydrite to hematite. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basavegowda, N.; Mishra, K.; Lee, Y.R. Synthesis, characterization, and catalytic applications of hematite (α-Fe2O3) nanoparticles as reusable nanocatalyst. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Asoufi, H.M.; Al-Antary, T.M.; Awwad, A.M. Green route for synthesis hematite (α-Fe2O3) nanoparticles: Toxicity effect on the green peach aphid, Myzus persicae (Sulzer). Environ. Nanotechnol. Monit. Manag. 2018, 9, 107–111. [Google Scholar]
- Lunin, A.V.; Kolychev, E.L.; Mochalova, E.N.; Cherkasov, V.R.; Nikitin, M.P. Synthesis of highly-specific stable nanocrystalline goethite-like hydrous ferric oxide nanoparticles for biomedical applications by simple precipitation method. J. Colloid Interface Sci. 2019, 541, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Sen, S.; Suja, G.; Senthil, S.L.; Kumar, T.V. Evaluation of cytotoxicity of hematite nanoparticles in bacteria and human cell lines. Colloids Surf. B 2017, 157, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Lunin, A.V.; Sokolov, I.L.; Zelepukin, I.V.; Zubarev, I.V.; Yakovtseva, M.N.; Mochalova, E.N.; Rozenberg, J.M.; Nikitin, M.P.; Kolychev, E.L. Spindle-like MRI-active europium-doped iron oxide nanoparticles with shape-induced cytotoxicity from simple and facile ferrihydrite crystallization procedure. RSC Adv. 2020, 10, 7301–7312. [Google Scholar] [CrossRef] [Green Version]
- Shevchenko, K.G.; Cherkasov, V.R.; Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Surface plasmon resonance as a tool for investigation of non-covalent nanoparticle interactions in heterogeneous self-assembly & disassembly systems. Biosens. Bioelectron. 2017, 88, 3–8. [Google Scholar]
- Cherkasov, V.R.; Mochalova, E.N.; Babenyshev, A.V.; Vasilyeva, A.V.; Nikitin, P.I.; Nikitin, M.P. Nanoparticle beacons: Supersensitive smart materials with on/off-switchable affinity to biomedical targets. ACS Nano 2020, 14, 1792–1803. [Google Scholar] [CrossRef]
- Sokolov, I.L.; Cherkasov, V.R.; Tregubov, A.A.; Buiucli, S.R.; Nikitin, M.P. Smart materials on the way to theranostic nanorobots: Molecular machines and nanomotors, advanced biosensors, and intelligent vehicles for drug delivery. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1530–1544. [Google Scholar] [CrossRef]
- Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Advanced Smart Nanomaterials with Integrated Logic-Gating and Biocomputing: Dawn of Theranostic Nanorobots. Chem. Rev. 2018, 118, 10294–10348. [Google Scholar] [CrossRef] [Green Version]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery: Via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Orlov, A.V.; Sokolov, I.L.; Minakov, A.A.; Nikitin, P.I.; Ding, J.; Bader, S.D.; Rozhkova, E.A.; Novosad, V. Ultrasensitive detection enabled by nonlinear magnetization of nanomagnetic labels. Nanoscale 2018, 10, 11642–11650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.; Tng, L.; Lim, T.; Choo, M.; Zhang, J.; Tan, H.R.; Bai, S. Hydrothermal synthesis of octadecahedral hematite (α-Fe 2O3) nanoparticles: An epitaxial growth from goethite (α-FeOOH). J. Phys. Chem. C 2014, 118, 10903–10910. [Google Scholar] [CrossRef]
- Echigo, T.; Monsegue, N.; Aruguete, D.M.; Murayama, M.; Hochella, M.F. Nanopores in hematite (α-Fe2O3) nanocrystals observed by electron tomography. Am. Mineral. 2013, 98, 154–162. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, M.P.; Shipunova, V.O.; Deyev, S.M.; Nikitin, P.I. Biocomputing based on particle disassembly. Nat. Nanotechnol. 2014, 9, 716–722. [Google Scholar] [CrossRef]
- Amnis Corporation. Image Data Exploration and Analysis Software User’s Manual Version 6.2; Amnis Corporation: Seattle, WA, USA, 2015. [Google Scholar]
- Baxter, A.E.; Russell, R.A.; Duncan, C.J.; Moore, M.D.; Willberg, C.B.; Pablos, J.L.; Finzi, A.; Kaufmann, D.E.; Ochsenbauer, C.; Kappes, J.C.; et al. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 2014, 16, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.N.; Sebastian, J.A.; Parsons, M.; Chang, T.C.; Acker, J.P.; Kolios, M.P. Application of Image Flow Cytometry for the Characterization of Red Blood Cell Morphology. In Proceedings of the SPIE, San Francisco, CA, USA, 22 February 2017. [Google Scholar]
- Mochalova, E.N.; Kotov, I.A.; Rozenberg, J.M.; Nikitin, M.P. Precise Quantitative Analysis of Cell Targeting by Particle-Based Agents Using Imaging Flow Cytometry and Convolutional Neural Network. Cytometry A 2020, 97, 279–287. [Google Scholar] [CrossRef]
- Rodrigues, M.A. Automation of the in vitro micronucleus assay using the Imagestream® imaging flow cytometer. Cytometry A 2018, 93, 706–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuba-Surma, E.K.; Kucia, M.; Abdel-Latif, A.; Dawn, B.; Hall, B.; Singh, R.; Lillard, J.W.; Ratajczak, M.Z. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J. Cell. Mol. Med. 2008, 12, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polikarpov, D.; Cherepanov, V.; Chuev, M.; Gabbasov, R.; Mischenko, I.; Nikitin, M.; Vereshagin, Y.; Yurenia, A.; Panchenko, V. Mössbauer evidence of 57Fe3O4 based ferrofluid biodegradation in the brain. Hyperfine Interact. 2014, 226, 421–430. [Google Scholar] [CrossRef]
- Morozov, V.N.; Kanev, I.L.; Mikheev, A.Y.; Shlyapnikova, E.A.; Shlyapnikov, Y.M.; Nikitin, M.P.; Nikitin, P.I.; Nwabueze, A.O.; van Hoek, M.L. Generation and delivery of nanoaerosols from biological and biologically active substances. J. Aerosol Sci. 2014, 69, 48–61. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunin, A.V.; Lizunova, A.A.; Mochalova, E.N.; Yakovtseva, M.N.; Cherkasov, V.R.; Nikitin, M.P.; Kolychev, E.L. Hematite Nanoparticles from Unexpected Reaction of Ferrihydrite with Concentrated Acids for Biomedical Applications. Molecules 2020, 25, 1984. https://doi.org/10.3390/molecules25081984
Lunin AV, Lizunova AA, Mochalova EN, Yakovtseva MN, Cherkasov VR, Nikitin MP, Kolychev EL. Hematite Nanoparticles from Unexpected Reaction of Ferrihydrite with Concentrated Acids for Biomedical Applications. Molecules. 2020; 25(8):1984. https://doi.org/10.3390/molecules25081984
Chicago/Turabian StyleLunin, Afanasy V., Anna A. Lizunova, Elizaveta N. Mochalova, Maria N. Yakovtseva, Vladimir R. Cherkasov, Maxim P. Nikitin, and Eugene L. Kolychev. 2020. "Hematite Nanoparticles from Unexpected Reaction of Ferrihydrite with Concentrated Acids for Biomedical Applications" Molecules 25, no. 8: 1984. https://doi.org/10.3390/molecules25081984