The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical and Technological Characterization of Nanosystems
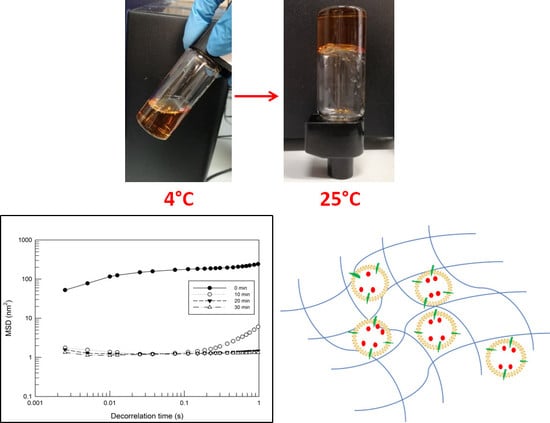
2.2. Gelation Temperature of Poloxamer Solutions
2.3. Microrheology Characterization by Rheolaser Master
2.4. Dynamic Rheological Characterization of Hydrogels
2.5. Percutaneous Permeation Studios of Paclitaxel Loaded-Ethosomes, Transfersomes and Niosomes, Included in Poloxamer Gel
3. Materials and Methods
3.1. Chemicals
3.2. Methods
3.2.1. Ethosomes Preparation
3.2.2. Transfersomes Preparation
3.2.3. Niosomes Preparation
3.2.4. Poloxamer 407-Based Hydrogel Preparation
3.2.5. Physicochemical Characterization of Vesicles Formulations
3.2.6. Determination of Gelation Temperature of Poloxamer 407-Hydrogles
3.2.7. Diffusing Wave Spectroscopy (DWS)
3.2.8. Hydrogel Dynamic Rheological Characterization
3.2.9. Preparation of Paclitaxel-Loaded TDDSs and Percutaneous Permeation Studies
3.2.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef] [PubMed]
- Dimer, F.A.; Pohlmann, A.R.; Guterres, S.S. Characterization of rheology and release profiles of olanzapine-loaded lipid-core nanocapsules in thermosensitive hydrogel. J. Nanosci. Nanotechnol. 2013, 13, 8144–8153. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharmaceut. Sci. 2006, 9, 339–358. [Google Scholar]
- Veyries, M.L.; Couarraze, G.; Geiger, S.; Agnely, F.; Massias, L.; Kunzli, B.; Faurisson, F. Controlled release of vancomycin from poloxamer 407 gels. Int. J. Pharm. 1999, 192, 183–193. [Google Scholar] [CrossRef]
- Shin, S.C.; Cho, C.W. Physicochemical Characterizations of Piroxicam-Poloxamer Solid Dispersion. Pharm. Dev. Technol. 1997, 2, 403–407. [Google Scholar] [CrossRef]
- Chi, S.C.; Jun, H.W. Release rates of ketoprofen from poloxamer gels in a membraneless diffusion cell. J. Pharm. Sci. 1991, 80, 280–283. [Google Scholar] [CrossRef]
- Shin, S.; Cho, C.; Oh, I. Enhanced efficacy by percutaneous absorption of piroxicam from the poloxamer gel in rats. Int. J. Pharm. 2002, 193, 213–218. [Google Scholar] [CrossRef]
- Cevc, G.; Schatzlein, A.G.; Richardsen, H.; Vierl, U. Overcoming Semipermeable Barriers, Such as the Skin, with Ultradeformable Mixed Lipid Vesicles, Transfersomes, Liposomes, or Mixed Lipid Micelles. Langmuir 2003, 19, 10753–10763. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Rel. 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwah, H.; Garg, T.; Rath, G.; Goyal, A.K. Development of transferosomal gel for trans-dermal delivery of insulin using iodine complex. Drug Deliv. 2016, 23, 1636–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentley, M.V.L.B.; Marchetti, J.M.; Ricardo, N.; Ali-Abi, Z.; Collet, J.H. Influence of lecithin on some physical chemical properties of poloxamer gels: Rheological, microscopic and in vitro permeation studies. Int. J. Pharm. 1999, 193, 49–55. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.J.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]
- Fang, Y.P.; Huang, Y.B.; Wu, P.B.; Tsai, Y.H. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur. J. Pharm. Biopharm. 2009, 73, 391–398. [Google Scholar] [CrossRef]
- Wang, W.; Liu, F.; Gao, Y. Quercetagetin loaded in soy protein isolate–κ-carrageenan complex: Fabrication mechanism and protective effect. Food Res. Int. 2016, 83, 31–40. [Google Scholar] [CrossRef]
- Paolino, D.; Lucania, G.; Mardente, D.; Alhaique, F.; Fresta, M. Ethosomes for skin delivery of ammonium glycyrrhizinate: In vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J. Control. Rel. 2005, 106, 99–110. [Google Scholar] [CrossRef]
- Celia, C.; Cilurzo, F.; Trapasso, E.; Cosco, D.; Fresta, M.; Paolino, D. Ethosomes® and transfersomes® containing linoleic acid: Physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. Microdevices 2012, 14, 119–130. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef]
- Ainbinder, D.; Paolino, D.; Fresta, M.; Touitou, E. Drug Delivery Applications with Ethosomes. J. Biomed. Nanotechnol. 2010, 6, 558–568. [Google Scholar] [CrossRef]
- Benson, H.A.E. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Rahimpour, Y.; Kouhsoltani, M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin. Drug Del. 2013, 10, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Girigoswami, A.; Das, S.; De, S. Fluorescence and dynamic light scattering studies of niosomes-membrane mimetic systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.S.; Choi, J.S.; Quan, Q.Z.; Rhee, J.D.; Kim, C.K.; Lim, S.J.; Kim, K.M.; Oh, P.S.; Choi, H.G. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001, 226, 195–205. [Google Scholar] [CrossRef]
- Choi, H.G.; Jung, J.H.; Ryu, J.M.; Yoon, S.J.; Oh, Y.K.; Kim, C.K. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int. J. Pharm. 1998, 165, 33–44. [Google Scholar] [CrossRef]
- Ceniti, C.; Froiio, F.; Britti, D.; Paolino, D.; Costanzo, N. Rheological characteristics of bovine colostrum and their correlation with immunoglobulin G. Int. J. Dairy Technol. 2019, 72, 345–349. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, S.; Zhang, H.; Cheng, Y.; Yin, L. Physical stability, microstructure and micro-rheological properties of water-in-oil-in-water (W/O/W) emulsions stabilized by porcine gelatin. Food Chem. 2018, 253, 63–70. [Google Scholar] [CrossRef]
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164. [Google Scholar] [CrossRef]
- Luo, J.; Wang, T.; Guo, H.; Ren, F. Effects of Size and Stability of Native Fat Globules on the Formation of Milk Gel Induced by Rennet. J. Food Sci. 2017, 82, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Huang, J.; Shao, H.; Hu, X.; Song, L.; Zhang, Y. Characterization of bladder acellular matrix hydrogel with inherent bioactive factors. Mater. Sci. Eng. C 2017, 77, 184–189. [Google Scholar]
- Sun, C.; Wu, T.; Liu, R.; Liang, B.; Tian, Z.; Zhang, E.; Zhang, M. Effects of superfine grinding and microparticulation on the surface hydrophobicity of whey protein concentrate and its relation to emulsions stability. Food Hydrocoll. 2015, 51, 512–518. [Google Scholar] [CrossRef]
- Degrand, L.; Michon, C.; Bosc, V. New insights into the study of the destabilization of oil-in-water emulsions with dextran sulfate provided by the use of light scattering methods. Food Hydrocoll. 2016, 52, 848–856. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, M.K.; Kim, M.H.; Kim, C.K. Effect of additives on the physicochemical properties of liquid suppository bases. Int. J. Pharm. 1999, 190, 13–19. [Google Scholar] [CrossRef]
- Mason, T.G.; Gang, H.; Weitz, D.A. Rheology of complex fluids measured by dynamic light scattering. J. Mol. Struct. 1996, 383, 81–90. [Google Scholar] [CrossRef]
- Kandemir, N.; Xia, Y.; Duan, P.; Yang, W.; Chen, J. Rheological Characterization of Agarose and Poloxamer 407 (P407) Based Hydrogels. MRS Adv. 2018, 3, 1719–1724. [Google Scholar] [CrossRef]
- Surapaneni, M.S.; Das, S.K.; Das, N.G. Designing Paclitaxel Drug Delivery Systems Aimed at Improved Patient Outcomes: Current Status and Challenges. ISRN Pharmacol. 2012, 2012, 623139. [Google Scholar] [CrossRef] [Green Version]
- Langer, R. Polymer-controlled drug delivery systems. Acc. Chem. Res. 1993, 26, 537–542. [Google Scholar] [CrossRef]
- Antunes, F.E.; Gentile, L.; Rossi, C.O.; Tavano, L.; Ranieri, G.A. Gels of Pluronic F127 and nonionic surfactants from rheological characterization to controlled drug permeation. Colloids Surf. B Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef]
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes: Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Sharma, V.; Sharma, M. Optimization, in vitro cytotoxicity and penetration capability of deformable nanovesicles of paclitaxel for dermal chemotherapy in Kaposi sarcoma. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1671–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schatzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta 1998, 1368, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Primavera, R.; Di Francesco, M.; De Cola, A.; De Laurenzi, V.; Paolino, D.; Ciancaioni, M.; Carafa, M.; Celia, C.; Di Stefano, A.; Fresta, M.; et al. HPLC–FLD and spectrofluorometer apparatus: How to best detect fluorescent probe-loaded niosomes in biological samples. Colloids Surf. B Biointerfaces 2015, 135, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Froiio, F.; Costanzo, N.; Poerio, A.; Lugli, M.; Fresta, M.; Britti, D.; Paolino, D. Effects of flour mean particle size, size distribution and water content on rheological properties of wheat flour doughs. Eur. Food Res. Technol. 2019, 245, 2053–2062. [Google Scholar] [CrossRef]
- Alcaro, S.; Ventura, C.A.; Paolino, D.; Battaglia, D.; Ortuso, F.; Cattel, L.; Puglisi, G.; Fresta, M. Preparation, characterization, molecular modeling and In vitro activity of paclitaxel—Cyclodextrin complexes. Bioorg. Med. Chem. Lett. 2002, 12, 1637–1641. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Ottimo, S.; Paolino, D.; Ferrero, A.; Dosio, F.; Castelli, F. Squalenoyl prodrug of paclitaxel: Synthesis and evaluation of its incorporation in phospholipid bilayers. Int. J. Pharm. 2012, 436, 135–140. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples are not available from the authors. |









| Sample | Mean Size (nm) | Polydispersity Index | Zeta-Potential (mV) |
|---|---|---|---|
| Ethosomes | 200.00 ± 4.43 | 0.16 ± 0.01 | −15.20 ± 0.38 |
| Transferosomes | 187.90 ± 1.87 | 0.24 ± 0.01 | −29.50 ± 0.59 |
| Niosomes | 123.50 ± 1.01 | 0.22 ± 0.01 | −26.00 ± 0.35 |
| Sample | Shear Rate (s−1) |
|---|---|
| 20% Poloxamer 407 | 0.1585 ± 0.0015 |
| 25% Poloxamer 407 | 0.2512 ± 0.0003 |
| 30% Poloxamer 407 | 0.6310 ± 0.0032 |
| Sample | Shear Viscosity (Pa·s) at Different Shear Rate | |||
|---|---|---|---|---|
| 0.1 s−1 | 1 s−1 | 10 s−1 | 100 s−1 | |
| 20% Poloxamer 407 | 1618000.0 ± 230.1 | 173.5 ± 10.6 | 19.7 ± 2.5 | 2.6 ± 0.6 |
| 25% Poloxamer 407 | 3577000.0 ± 307.6 | 434.3 ± 7.9 | 52.8 ± 1.6 | 6.3 ± 0.9 |
| 30% Poloxamer 407 | 5708000.0 ± 98.7 | 610.1 ± 24.0 | 75.9 ± 2.0 | 9.7 ± 1.0 |
| Sample | Mean Size (nm) | Polydispersity Index | EE (%) |
|---|---|---|---|
| Ethosomes | 309.00 ± 2.51 | 0.19 ± 0.01 | 65.54 ± 1.47 |
| Transferosomes | 265.07 ± 19.00 | 0.56 ± 0.01 | 57.27 ± 1.03 |
| Niosomes | 218.50 ± 7.53 | 0.32 ± 0.01 | 42.5 ± 0.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules 2020, 25, 1979. https://doi.org/10.3390/molecules25081979
Cristiano MC, Froiio F, Mancuso A, De Gaetano F, Ventura CA, Fresta M, Paolino D. The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel. Molecules. 2020; 25(8):1979. https://doi.org/10.3390/molecules25081979
Chicago/Turabian StyleCristiano, Maria Chiara, Francesca Froiio, Antonia Mancuso, Federica De Gaetano, Cinzia Anna Ventura, Massimo Fresta, and Donatella Paolino. 2020. "The Rheolaser Master™ and Kinexus Rotational Rheometer® to Evaluate the Influence of Topical Drug Delivery Systems on Rheological Features of Topical Poloxamer Gel" Molecules 25, no. 8: 1979. https://doi.org/10.3390/molecules25081979